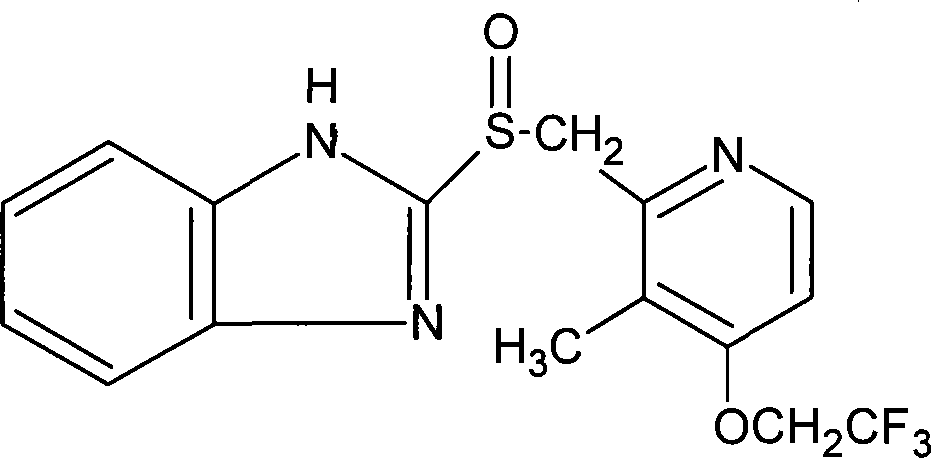
Lansoprazole intestine solution capsule and its preparation method
A technology of lansoprazole enteric solution and lansoprazole is applied in the field of lansoprazole enteric solution liquid capsule and its manufacture, which can solve the problem of unstable raw material temperature and humidity of lansoprazole, inability to directly fill hollow capsules, Serious environmental pollution and other problems, to achieve the effects of good product quality stability, avoiding oxygen and moisture contact, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The lansoprazole raw material is pulverized, passed through a 200-mesh sieve for subsequent use, pregelatinized starch, polyoxyethylene 35 castor oil, magnesium oxide, poloxamer, sodium citrate, and gum arabic are distributed and pulverized, and passed through a 150-mesh sieve for subsequent use.
[0029] Take by weighing lansoprazole 150g, pregelatinized starch 300g, pulverize after mixing; Take by weighing camellia oil 1000g, polyoxyethylene 35 castor oil 150g, magnesium oxide 50g, poloxamer (188) 100g, sodium citrate 150g, Gum Arabic 100g, after mixing, add the mixture of lansoprazole and pregelatinized starch, grind in colloid mill after mixing, obtain lansoprazole medicine;
[0030] Add 150g of polyacrylic acid resin to dissolve in 1000ml of ethanol to form a solution, which is used as the capsule sealing material.
[0031] According to the content determination method in the national quality standard, measure the content of the active ingredients of the above drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com