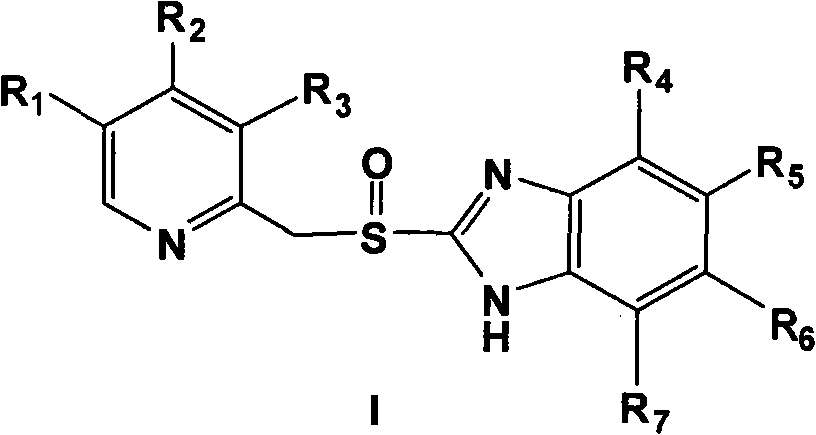
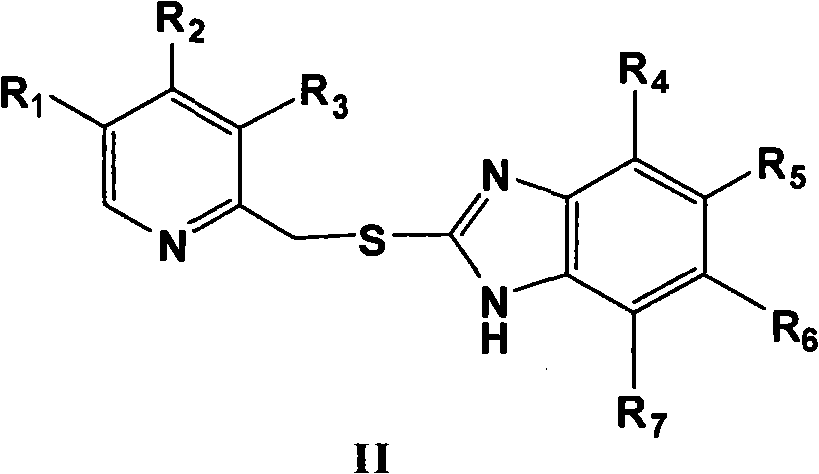
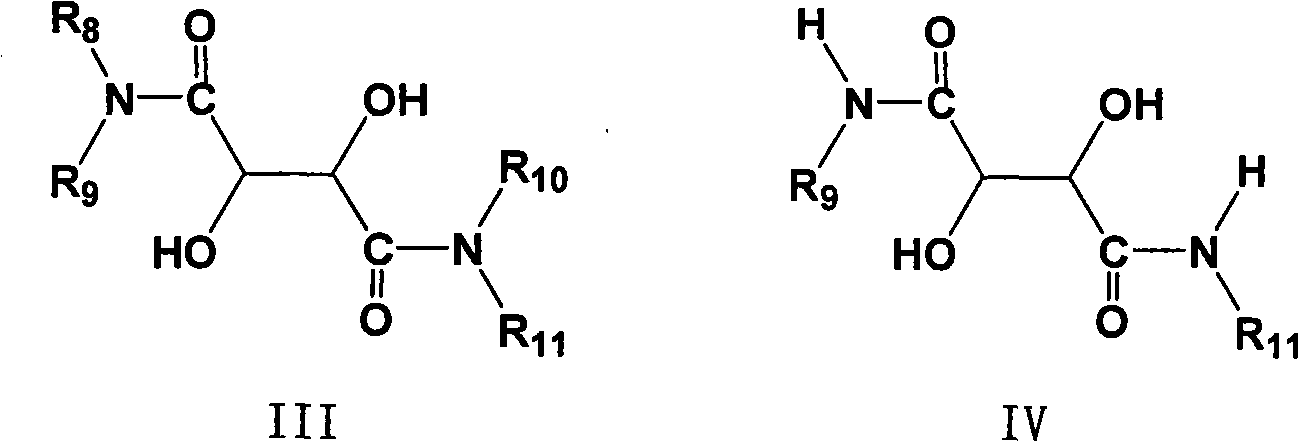
Novel method for preparing chiral sulphoxide compound
A technology for compounds and titanium compounds, applied in the field of chiral sulfoxide compounds, can solve the problems of no enantioselective oxidation of thioethers, no disclosure of a single enantiomer, etc., and achieve the effects of simple method and high optical purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 The effect of the structure of the chiral tartrate diamide bidentate ligand on the enantioselective catalytic oxidation reaction:
[0046] 0.31 g (0.95 mmol) of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]-1H-benzo Imidazole was dissolved in 2.5 ml of toluene with stirring at 70°C. Add 0.264 g (1.14 mmol) tartrate diamide at 60° C., and stir for 15 minutes. 0.16 g (0.57 mmol) of titanium tetraisopropoxide was added under heat preservation, and after heat preservation and stirring for 1 hour, 4.4 mg (0.24 mmol) of water was added, and heat preservation and stirring were carried out for 20 minutes. The temperature was lowered to 0°C, and 0.2 ml (1.05 mmol) of phenylisopropyl hydroperoxide was added dropwise. React at 0°C for 24 hours, add 50 ml of 10% sodium hydroxide solution and shake several times, then wash the aqueous solution twice with toluene, adjust the pH value of the aqueous layer to 7-8 with glacial acetic acid, extract with dichlor...
Embodiment 2
[0050] The influence of embodiment 2 additives on the enantioselective catalytic oxidation reaction:
[0051] 0.31 g (0.95 mmol) of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]-1H-benzimidazole Dissolve in 2.5 ml of toluene with stirring at 70°C. Add 0.132 g (0.57 mmol) of D-di-n-propionamide tartrate at 60° C., and stir for 15 minutes. 0.08 g (0.285 mmol) of titanium tetraisopropoxide was added under heat preservation, and after stirring for 1 hour, 2.2 mg (0.1 mmol) of water was added and stirred at 60° C. for 20 minutes. Cool down to 30°C, add 0.285 mmol of additives, keep stirring for 10 minutes, then cool down to 0°C, add 0.2 ml (1.05 mmol) of phenylisopropyl hydroperoxide dropwise. React at 0°C for 24 hours, add 50 ml of 10% sodium hydroxide solution and shake several times, then wash the aqueous solution twice with toluene, adjust the pH value of the aqueous layer to 7-8 with glacial acetic acid, extract with dichloromethane, The organic phase was was...
Embodiment 3
[0055] The enantioselective catalytic oxidation reaction under the room temperature condition of embodiment 3:
[0056] 0.31 g (0.95 mmol) of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]-1H-benzimidazole Dissolve in 2.5 ml of toluene with stirring at 70 °C. Add 0.132 g (0.57 mmol) di-n-propionamide tartrate at 60°C, stir for 15 minutes, then add 0.08 g (0.285 mmol) titanium tetraisopropoxide, keep stirring for 1 hour, then add 2.2 mg (0.1 mg) mol) water, stirred at 60°C for 20 minutes. Cool down to 30°C, add 0.285 mmol of additives, stir for 10 minutes, and then add dropwise 0.2 ml (1.05 mmol) of phenylisopropyl hydroperoxide. After reacting at 30°C for 5 hours, add 50 ml of 10% sodium hydroxide solution and shake several times, then wash the aqueous solution twice with toluene, adjust the pH value of the aqueous layer to 7-8 with glacial acetic acid, and extract with dichloromethane , and then washed the organic phase twice with saturated saline, dried over...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com