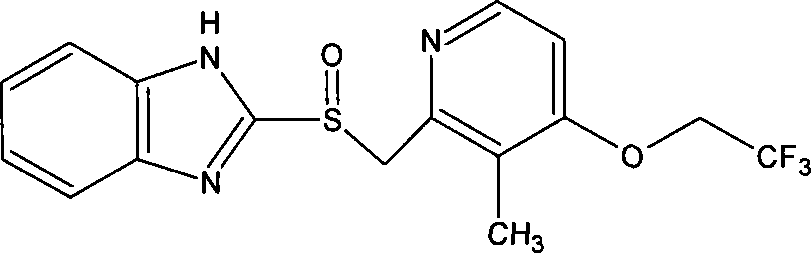
Lansoprazole freeze-dried powder for injection and preparing method thereof
A technology of lansoprazole and freeze-dried powder, applied in the field of medicine, can solve problems such as insufficient formation of excipients, incomplete appearance, etc., and achieve the effects of increasing sublimation efficiency, plump appearance and low water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
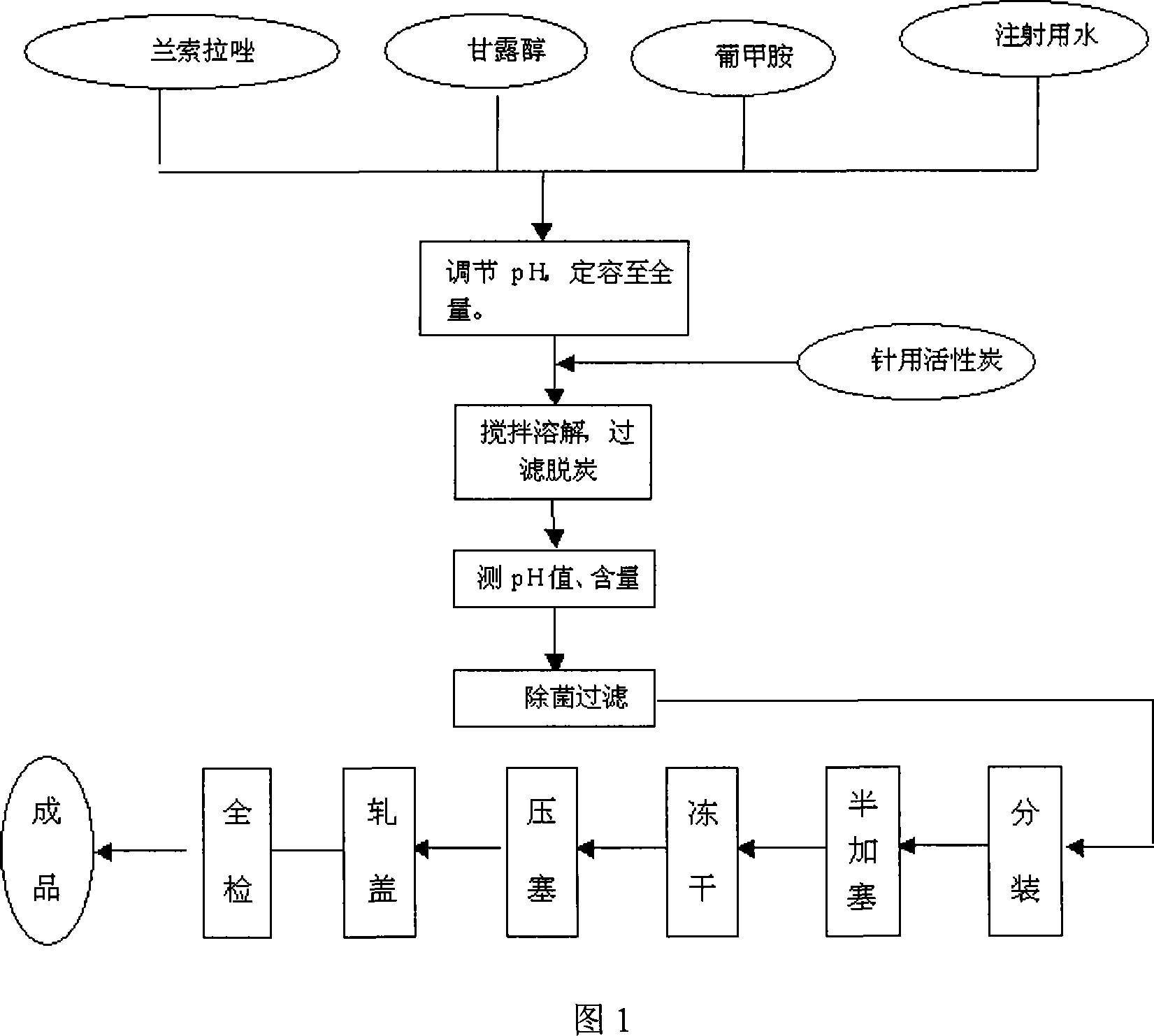
[0045] Add 30g of lansoprazole, 10g of meglumine and 200g of mannitol into the liquid mixing tank, add 2500ml of water for injection and stir until completely dissolved, adjust the pH to 10-12 with sodium hydroxide, add water for injection to 3000ml, and add to the prepared Add 0.1% medical activated carbon to the liquid medicine, stir at room temperature for 15 minutes, filter and decarburize, and then fine filter the filtrate with a 0.22 μm sterile microporous filter membrane, measure the pH value and content of the filtrate, divide it into 1000 bottles, and half stoppered, Cool the subpackaged medicinal solution to -48°C at a rate of 1.1°C / min, keep it warm and freeze for 3 hours, open the vacuum valve to evacuate to 15Pa, then slowly raise the temperature to -20°C within 8 hours at a uniform speed, and keep it dry for 1 hour. Then the temperature was raised to 5°C at a uniform rate within 5 hours, and then the liquid was heated to 40°C at a constant rate within 4 hours, kep...
Embodiment 2
[0047] Add 30g of lansoprazole, 10g of meglumine and 180g of mannitol into the liquid mixing tank, add 2500ml of water for injection and stir until completely dissolved, adjust the pH to 10-11 with sodium hydroxide, add water for injection to 3000ml, and add to the prepared Add 0.1% medical activated carbon to the liquid medicine, stir at room temperature for 15 minutes, filter and decarburize, and then fine filter the filtrate with a 0.22 μm sterile microporous filter membrane, measure the pH value and content of the filtrate, divide it into 1000 bottles, and half stoppered, Cool the subpackaged medicinal solution to -50°C at a rate of 1.2°C / min, keep it warm and freeze for 3 hours, open the vacuum valve to evacuate to 15Pa, then slowly heat up to -20°C at a uniform speed within 9 hours, and keep it dry for 2 hours. Then, the temperature was raised to 3°C at a uniform rate within 6 hours, and then the liquid was heated to 40°C at a constant rate within 4 hours, kept warm and d...
Embodiment 3
[0049] Add 30g of lansoprazole, 10g of meglumine and 220g of mannitol into the liquid mixing tank, add 2500ml of water for injection and stir until completely dissolved, adjust the pH to 11-12 with sodium hydroxide, add water for injection to 3000ml, and add to the prepared Add 0.1% medical activated carbon to the liquid medicine, stir at room temperature for 15 minutes, filter and decarburize, and then fine filter the filtrate with a 0.22 μm sterile microporous filter membrane, measure the pH value and content of the filtrate, divide it into 1000 bottles, and half stoppered, Cool the subpackaged medicinal solution to -46°C at a rate of 1°C / min, keep it warm and freeze for 3 hours, open the vacuum valve to evacuate to 15Pa, then slowly raise the temperature to -19°C within 7 hours at a uniform speed, and keep it dry for 1.5 hours. Then, within 4 hours, the temperature was raised to 4°C at a constant speed, and then the liquid was heated to 40°C within 4 hours, and then dried fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com