Separation and detection method of lansoprazole nitrogen oxide impurities
A technology for the separation of lansoprazole nitrogen oxides and methods, applied in the field of pharmaceutical analysis, can solve problems such as difficult to distinguish peak signals, inability to separate and detect nitrogen oxide impurities, and inability to reflect the purity of the main peak, so as to achieve improved separation and accurate purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example discloses the separation and detection method of rryazole oxide impurities.
[0041] A moisture mixed-like (commercially available) of about 10 mg is about 10 mg of methacolic acid, which is dissolved and diluted to the scale of methanol-water (60:40) solution in a 20 ml amount bottle. 10 μl of injection of octadecyl silica biless silica gel as a column (specification: 4.6 mm × 250 mm, 5 μm), with methanol to flow phase A, with volume ratio (100: 440: 4.4: 0.9) The methanol-water-triethylamine-phosphoric acid solution is a mobile phase B, and the pH is adjusted to 7.1 with a phosphoric acid solution (1 → 10), the detection wavelength is 284 nm, the injection volume is 5 μL, the column temperature is 35 ° C, according to Table 1 Shuttling is eluted.
[0042] Table 1
[0043]
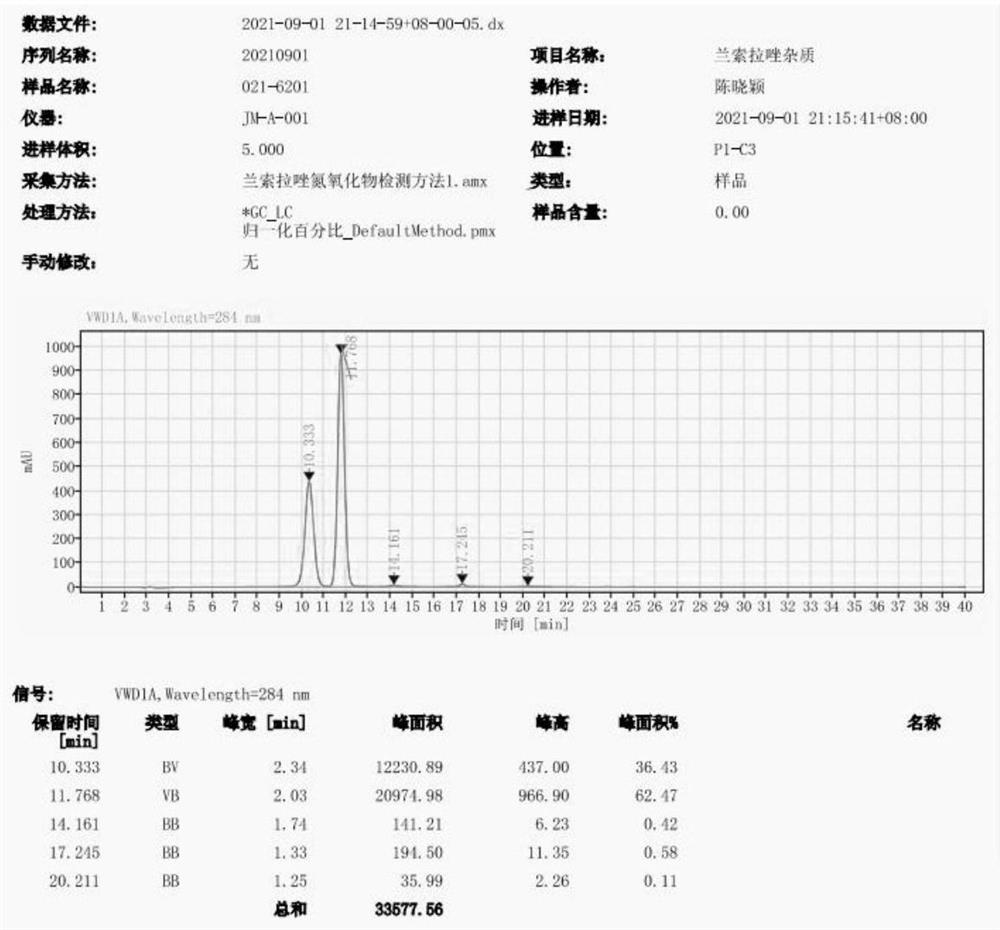
[0044] Observing HPLC map, see figure 1 : The retention time of Lamovazole impurities A is 10.356 min, and the retention time of Lamovazole nitrogen oxide impurities is 11.787 min.
Embodiment 2
[0046] This example discloses the separation and detection method of rryazole oxide impurities.
[0047]The difference from Embodiment 1 is that the elution gradient is Table 2.
[0048] Table 2
[0049]
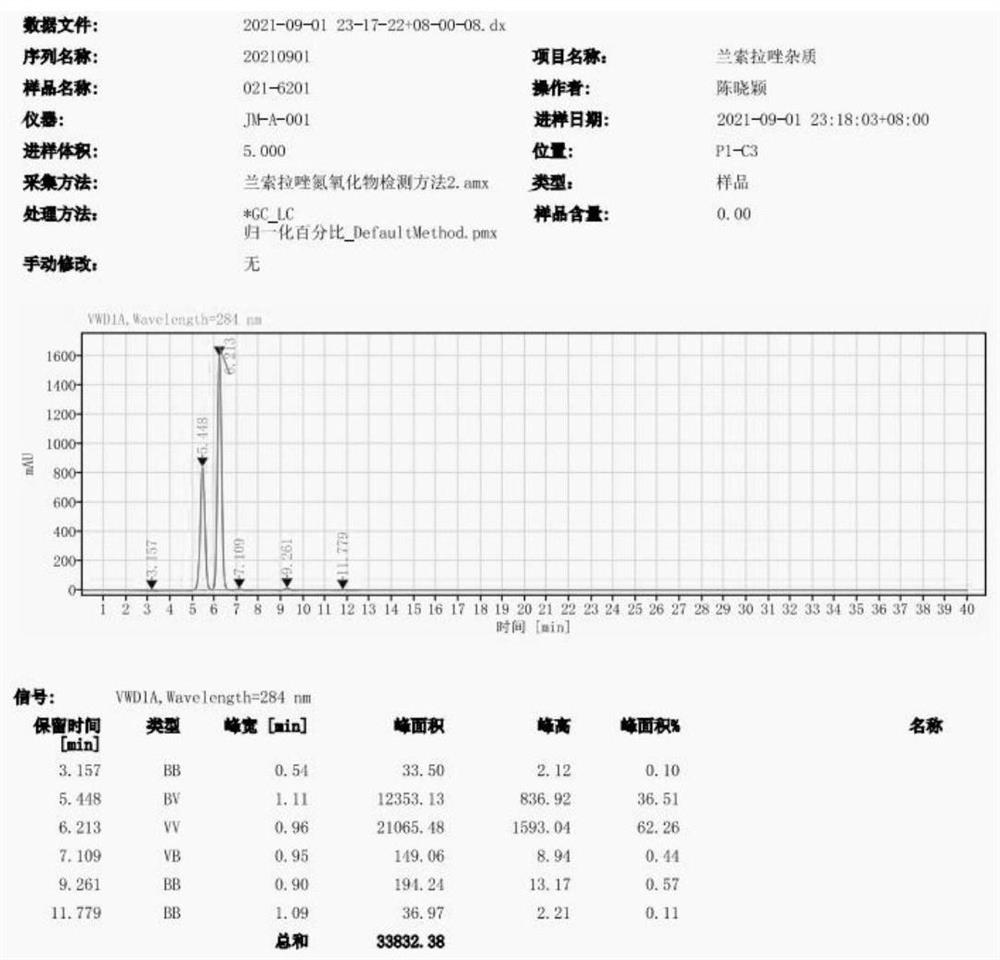
[0050] Observing HPLC map, see figure 2 : The retention time of Lan Solarazole impurities A is 5. minutes, and the retention time of Lamovazole nitrogen oxide impurities is 6.231 min.
Embodiment 3
[0052] This example discloses the separation and detection method of rryazole oxide impurities.
[0053] The difference from Example 1 is that the elution gradient is Table 3.
[0054] table 3
[0055]
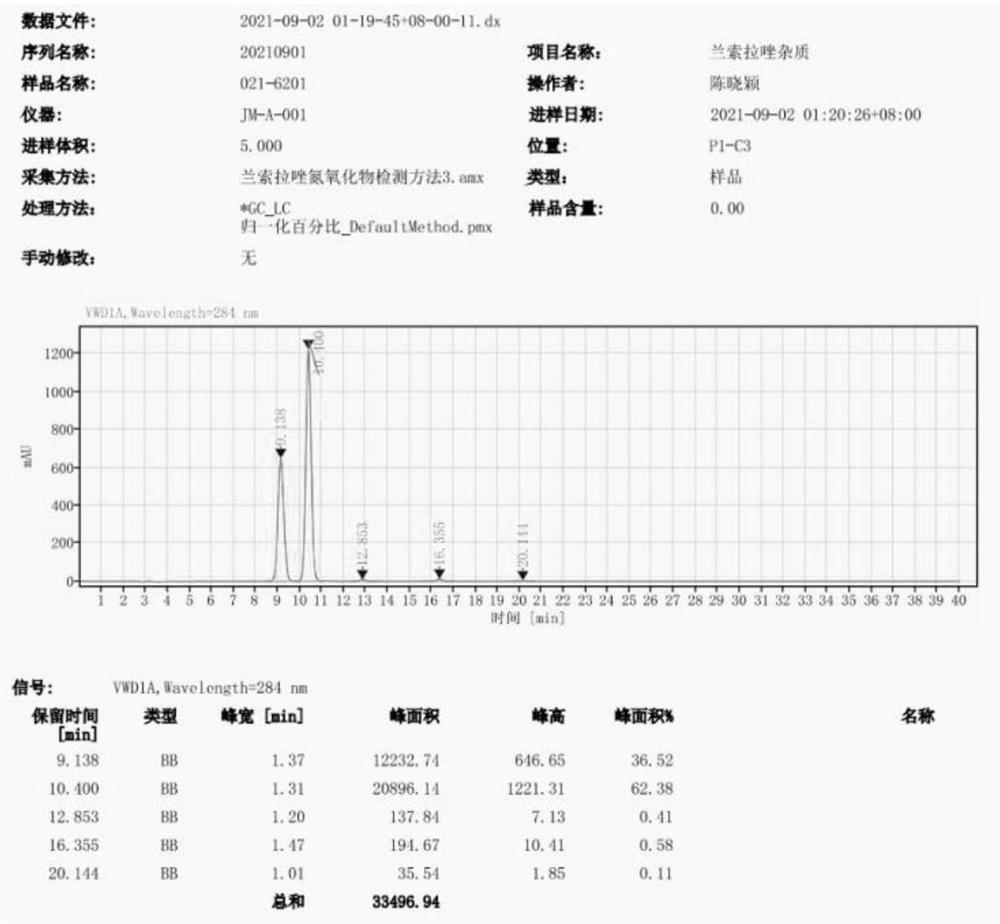
[0056] Observing HPLC map, see image 3 : The retention time of Lamovazole impurities A is 9.138 min, and the retention time of the Lamovazole nitrogen oxide impurities is 10.400 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com