A method for simultaneous detection of statin side chain and its isomer impurities
A technology of isomers and side chains, applied in the field of simultaneous detection of statin side chains and their isomer impurities, can solve the problems of low response and difficult detection, and achieve high sensitivity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
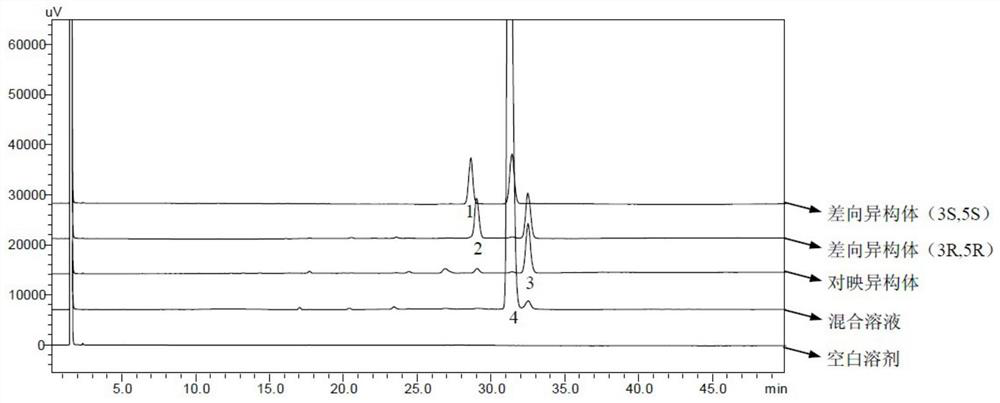
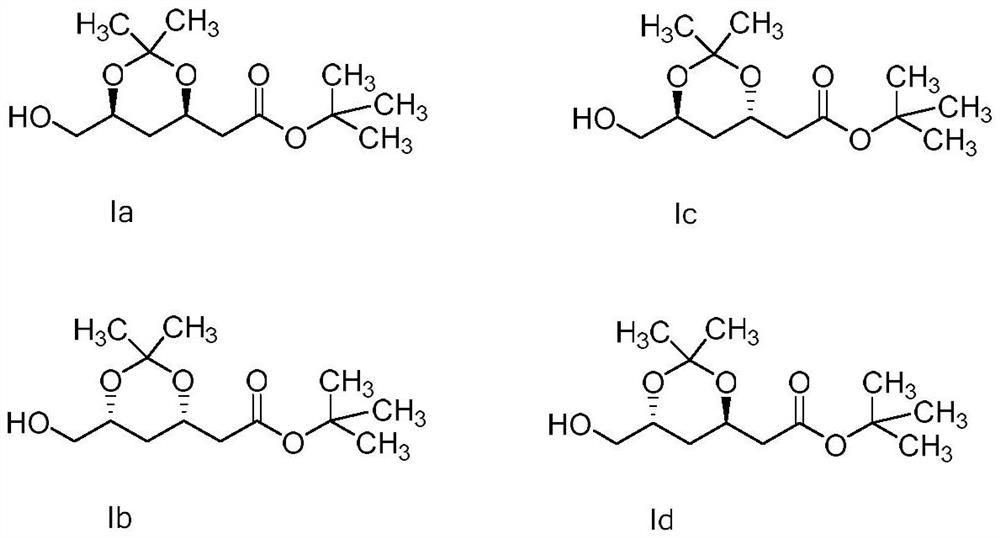
[0025] Statin side chain I is commonly used as the starting material of rosuvastatin, pitavastatin, fluvastatin, etc. There are four isomers, the structure is as follows, Ia (starting material) and Ib are enantiomers of each other, Ic and Id are the epimers of Ia, the configuration of Ia is the configuration required for the side chain in statin synthesis, and the other three are generally considered as impurities. The impurities in the side chain directly affect the impurities of the enantiomers and epimers of the final raw materials, and it is necessary to control them, first of all, a sensitive and accurate analysis method is required. According to its structural formula, it is an alcohol, and the response of the four isomers on the ultraviolet detector is very low, making it difficult to detect.
[0026]
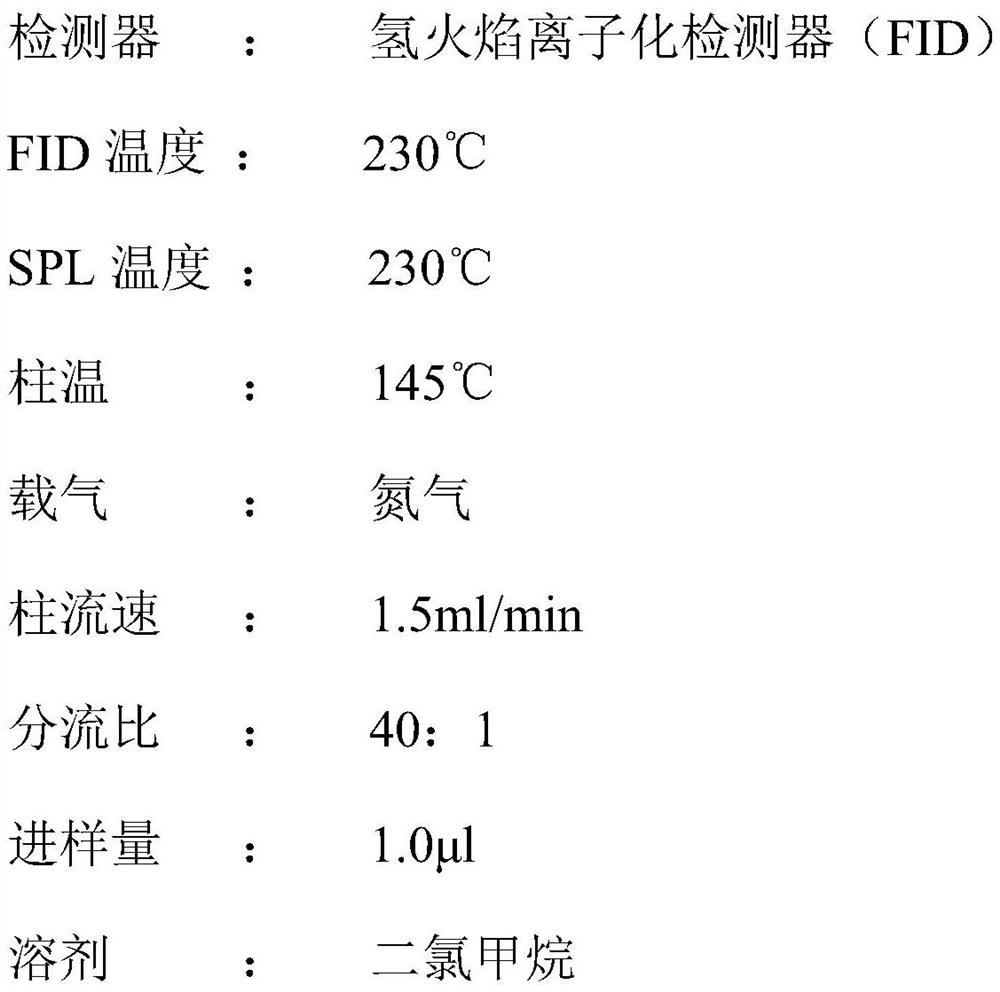
[0027] A method for simultaneously detecting statin side chains and its isomer impurities, the steps comprising: injecting a system suitability solution and the test ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com