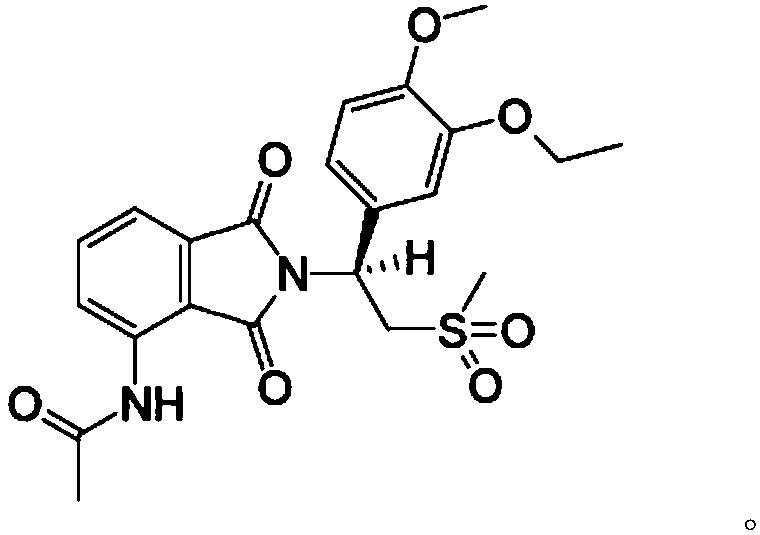
Apremilast solid composition and preparation method thereof
A solid composition and coating material technology, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of low bioavailability, dissolution, limited bioavailability, poor solubility of active ingredients, etc., and achieve bioavailability High, extensive clinical application prospects, and stable quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
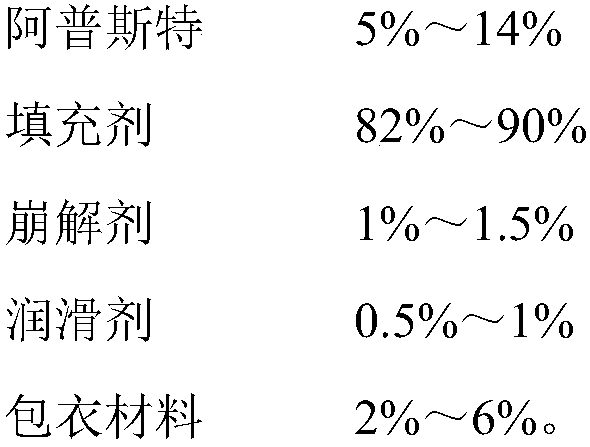
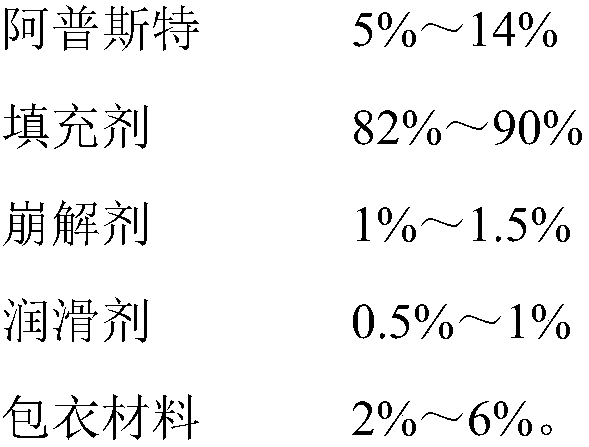
[0023] Plain Tablet Prescription:
[0024]
[0025] Coating Solution Prescription:
[0026] 3g
[0027] water 32g
[0028] Preparation:
[0029] ① Grind the prescribed amount of Apremilast and pass through a 250-mesh sieve; respectively pass through a 80-mesh sieve for lactose, microcrystalline cellulose, croscarmellose sodium, and micropowder silica gel;
[0030] ②Mix the above-mentioned Apremilast with lactose, microcrystalline cellulose, croscarmellose sodium, and micropowder silica gel for 20 minutes;
[0031] ③Transfer the uniformly mixed medicinal powder to a powder feeder and press into tablets;
[0032] ④The prescription amount It is dissolved in water and made into a solution of about 9% as a film coating solution to coat the drug-containing tablet core. During the coating process, the temperature of the material is controlled at 40°C to 42°C. After the process is completed, the weight of the tablet core increases by about 3%.
Embodiment 2
[0034] Plain Tablet Prescription:
[0035]
[0036] Coating Solution Prescription:
[0037] 3g
[0038] water 32g
[0039] Preparation:
[0040] ①Crush the prescription amount of Apremilast and pass through a 250-mesh sieve; lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate are respectively passed through a 80-mesh sieve for later use;
[0041] ②Mix the above-mentioned Apremilast with lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate for 10 minutes;
[0042] ③Transfer the uniformly mixed medicinal powder to a powder feeder and press into tablets;
[0043] ④The prescription amount It is dissolved in water and made into a solution of about 9% as a film coating solution to coat the drug-containing tablet core. During the coating process, the temperature of the material is controlled at 40°C to 42°C. After the process is completed, the weight of the tablet core increases by about 3%.
Embodiment 3
[0045] Plain Tablet Prescription:
[0046]
[0047] Coating Solution Prescription:
[0048] 3g
[0049] water 32g
[0050] Preparation:
[0051] ①Crush the prescription amount of Apremilast and pass through a 250-mesh sieve; lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate are respectively passed through a 80-mesh sieve for later use;
[0052] ④ Mix the above-mentioned Apremilast with lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate for 30 minutes;
[0053] ③Transfer the uniformly mixed medicinal powder to a powder feeder and press into tablets;
[0054] ④The prescription amount It is dissolved in water and made into a solution of about 9% as a film coating solution to coat the drug-containing tablet core. During the coating process, the temperature of the material is controlled at 40°C to 42°C. After the process is completed, the weight of the tablet core increases by about 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com