New apremilast crystal form H and preparation method thereof
A technology of crystal form and characteristic peaks, applied in the field of medicinal chemistry, to achieve the effect of good stability and strong solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of crystal form H
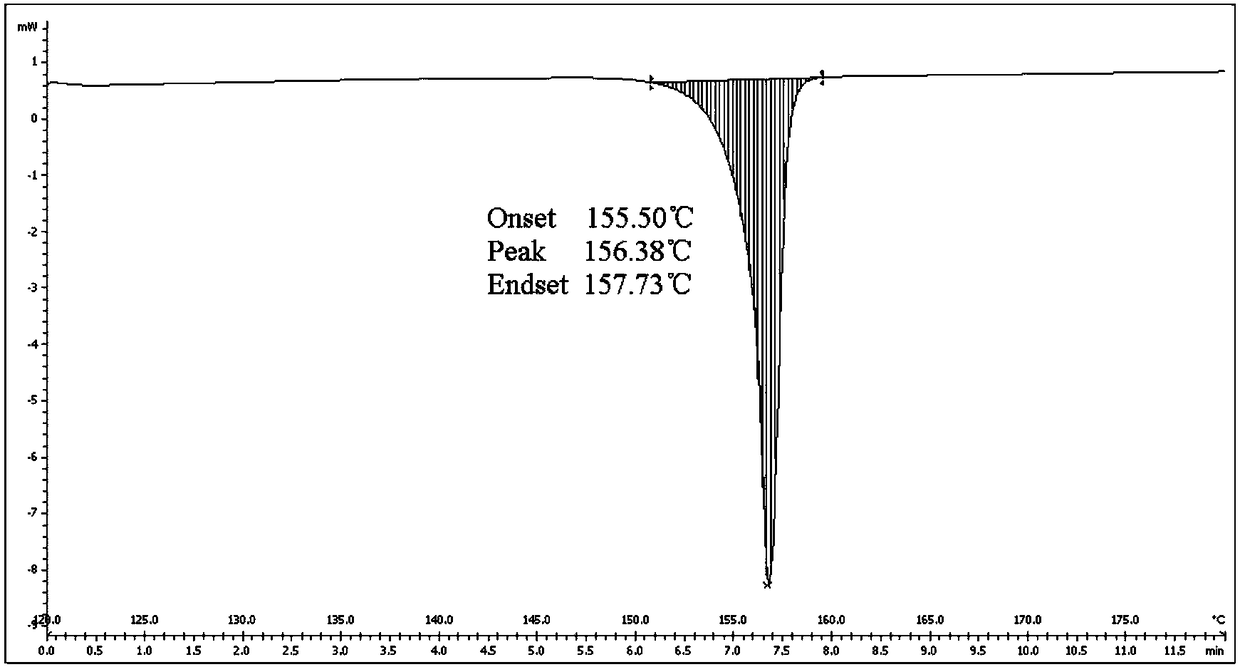
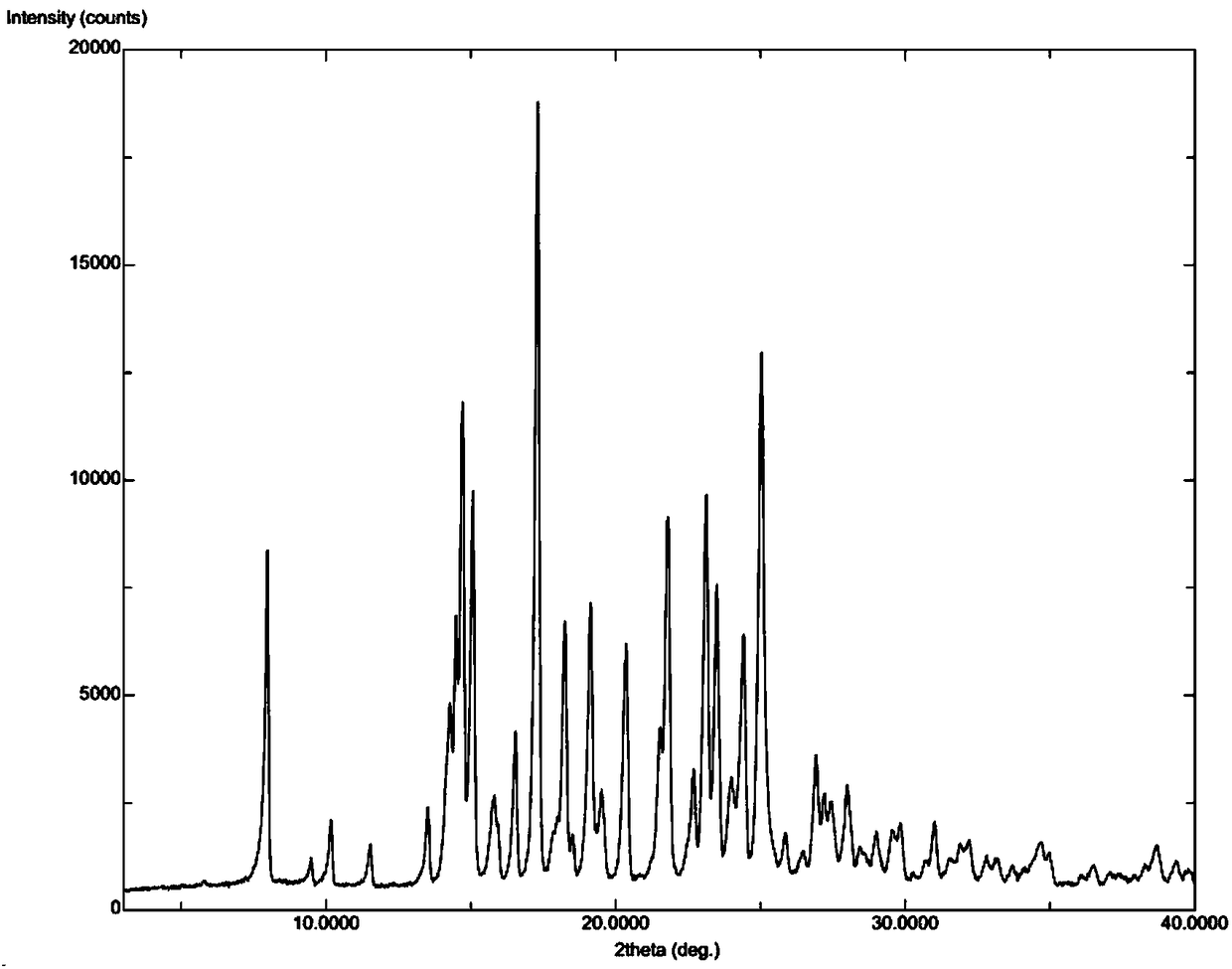
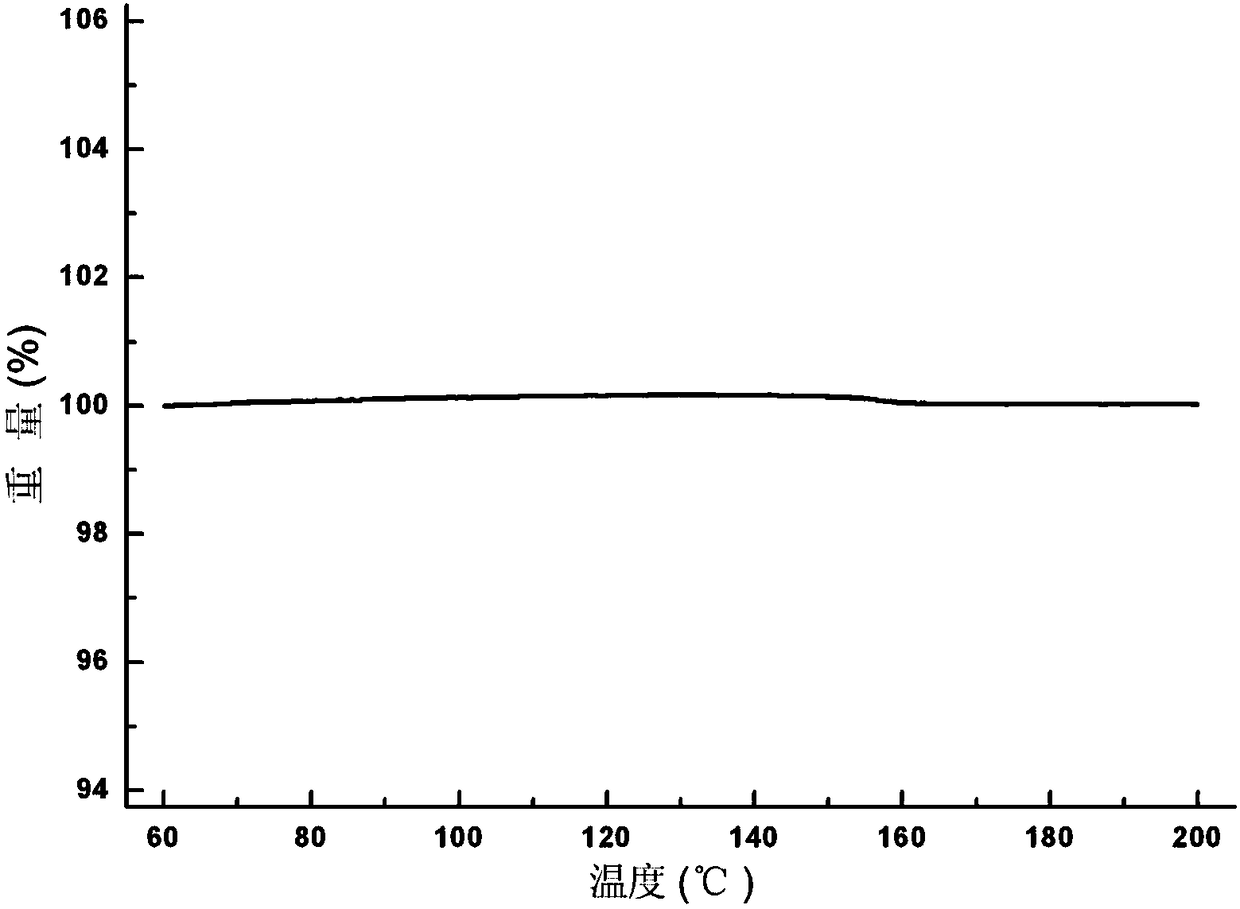
[0037] Put 5.0 g of Apremilast and 150 ml of absolute ethanol into a 250 ml three-neck flask, continue to stir and heat to 78° C. to reflux to dissolve the liquid, and the stirring rate is 200 r / min. After refluxing for 10 minutes, start to lower the temperature of the system to 10°C at a cooling rate of 10°C / h, grow crystals at this temperature for 2 hours, filter, and dry the solid at 50°C with air blast to constant weight. The molar yield of the process is 90.5%, and the measured HPLC purity is 99.96%. The results of X-ray powder diffraction and differential scanning calorimetry analysis showed that the solid was apremilast crystal form H.
Embodiment 2
[0038] Embodiment 2: Preparation of crystal form H
[0039] Put 5.0 g of Apremilast, 130 ml of acetone, and 130 ml of purified water into a 500 ml three-neck flask, continue stirring and heating to 50°C to dissolve, and the stirring rate is 300 r / min. Continue stirring at constant temperature for 10 min after dissolving. Afterwards, the temperature of the system was lowered to 5°C, the cooling rate was 30°C / h, and the crystal was grown at this temperature for 3 hours, filtered, and the solid was air-dried at 50°C until constant weight. The molar yield of the process is 72.1%, and the measured HPLC purity is 99.95%. The results of X-ray powder diffraction and differential scanning calorimetry analysis showed that the solid was apremilast crystal form H.
Embodiment 3
[0040] Embodiment 3: the preparation of crystal form H
[0041] Put 5.0 g of Apremilast and 300 ml of isopropanol into a 500 ml three-necked flask, continue stirring and heating to 75°C to dissolve, and the stirring rate is 300 r / min. Continue stirring at constant temperature for 10 min after dissolving. Then cool the system down to 50°C at a cooling rate of 5°C / h, then add the solid form containing crystal form H as a seed crystal to induce crystallization, and continue to grow crystals at this temperature for 3 hours, filter, and blast the solid at 50°C Dry to constant weight. The molar yield of the process is 81.0%, and the measured HPLC purity is 99.96%. The results of X-ray powder diffraction and differential scanning calorimetry analysis showed that the solid was apremilast crystal form H.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com