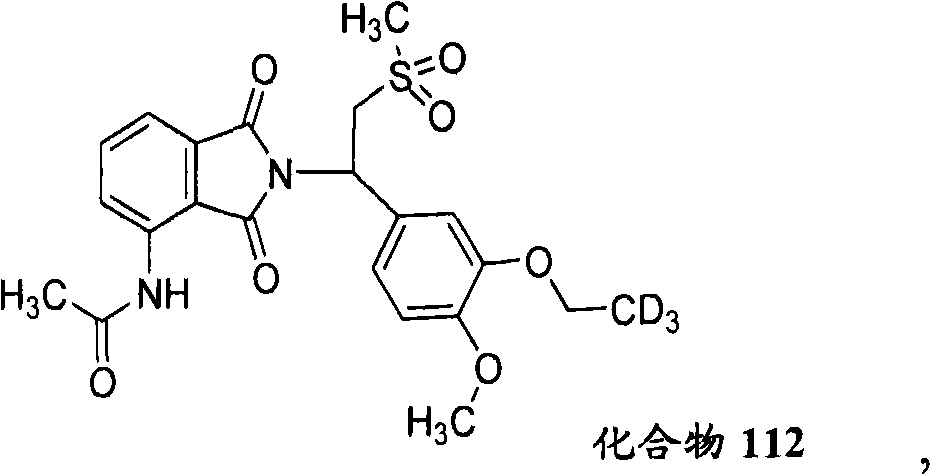
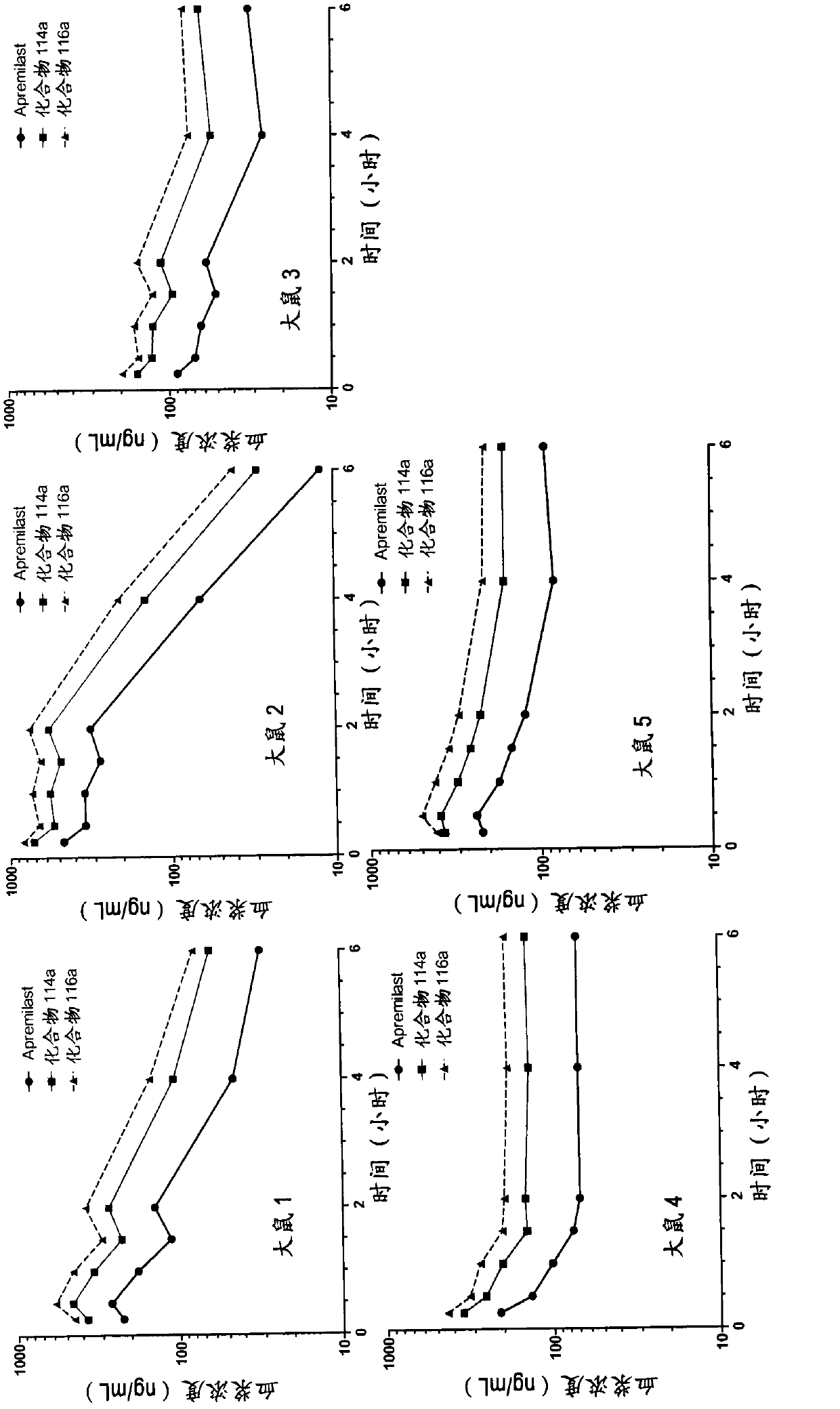
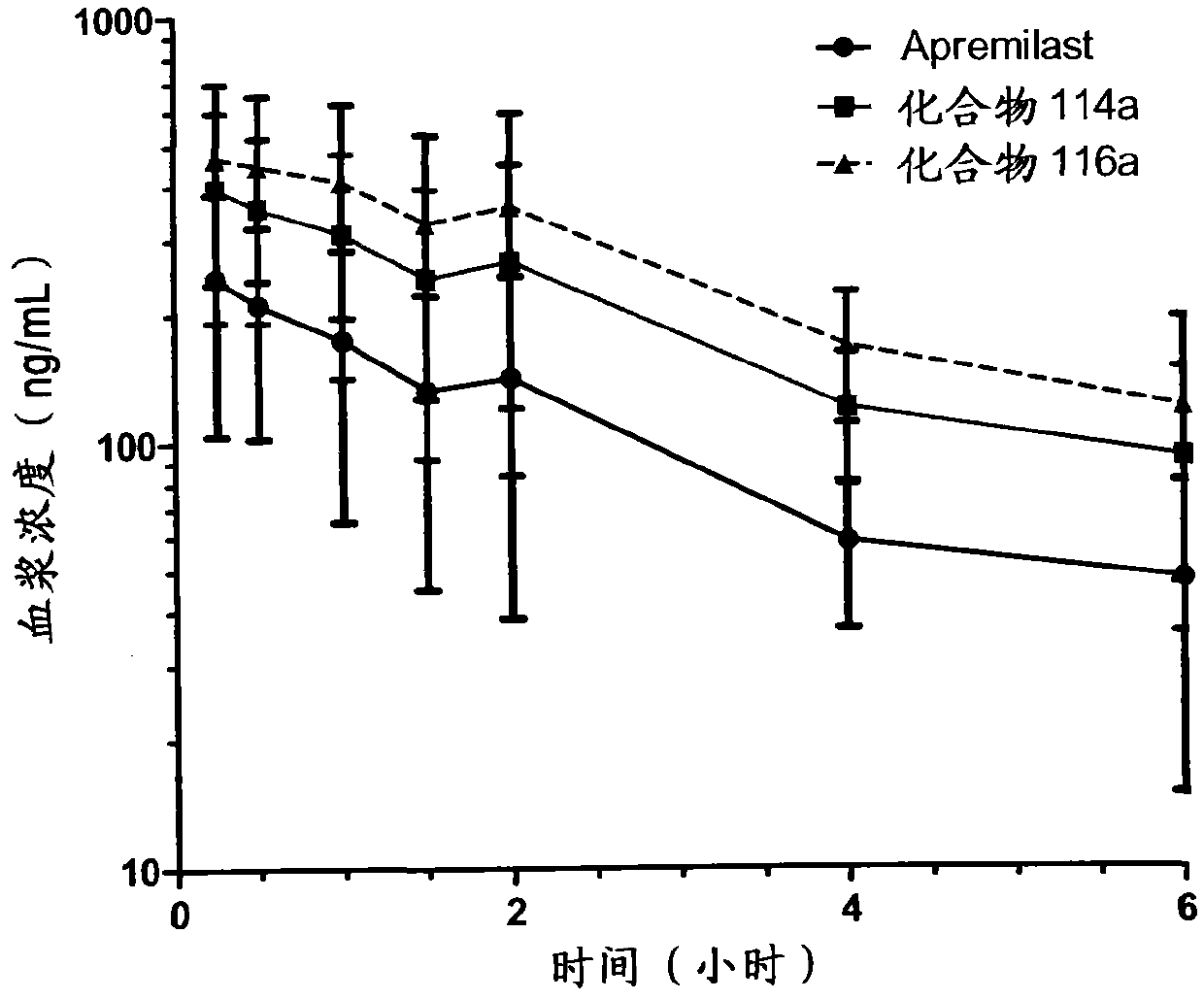
Substituted isoindoline-1,3-dione derivative
A technology of deuterium substitution and compound, which is applied in the direction of metabolic diseases, drug combination, and resistance to vector-borne diseases, etc., and can solve problems such as decreased metabolic clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] Scheme 2 describes the preparation of aldehyde 10, a useful starting material for Scheme 1. As generally described by Li, Juren et al. Hecheng Huaxue (1993), 1(4), 333-40, treatment of appropriately deuterated diol 13 with appropriately deuterated bromoethane 14 under phase transfer conditions yields phenol 15 . Reimer-Tiemann reaction of phenol 15 with chloroform yields aldehyde 16. Deuterated reagents and solvents can be used in this step to maximize the level of isotope incorporation. Alternatively, tetrabutylammonium bromide conditions can be used to convert 15 to 16 as generally described by Li, Ying-chun et al., Yingyong Huagong (2004), 33(1), 26-27. Treatment of 16 with appropriately deuterated dimethyl sulfate 17 yields the desired intermediate 10 according to the general method of Kiehlmann, E. et al., Organic Preparations and Procedures International (1982), 14(5), 337-42.
[0108] For example, commercially available dimethyl sulfate-d6 can be used as reage...
Embodiment 1
[0158] Example 1: (S)-N-(2-(1-d-2-(methylsulfonyl)-1-(3-(ethoxy-d 5 )-4-(methoxy-d 3 )Synthesis of phenyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide (compound 113a).
[0159] Scheme 4: Preparation of compound 113a.
[0160]
[0161] Step 1: 3-Hydroxy-4-(methoxy-d 3 )-ethyl benzoate (23).
[0162] Commercially available ester 22 (10 g, 55 mmol) was mixed with CD in DMF 3 I (99 atomic % D, Cambridge Isotopes; 8.1 g, 55 mol) and K 2 CO 3 (7.59g) were combined and stirred at room temperature over the weekend. LCMS showed 3 peaks with masses consistent with the starting material (20%), the desired mono-alkylated product 23 (55%) and the di-alkylated by-product (23%). The reaction was filtered through a pad of Celite, washed with EtOAc, and the filtrate was concentrated to almost dryness. The residue was dissolved in CH 2 Cl 2 (300mL), and the solution was washed with water (5x50mL), brine, dried (Na 2 SO 4 ) and concentrate. The crude product was purified by...
Embodiment 2
[0175] Example 2: (S)-N-(2-(2-(-(methylsulfonyl)-1-(3-(ethoxy-d 5 )-4-(methoxy-d 3 )Synthesis of phenyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide (compound 107a).
[0176] Scheme 5: Preparation of compound 107a.
[0177]
[0178]
[0179] Step 1: 3-Hydroxy-4-(methoxy-d 3 )-benzaldehyde (27).
[0180] Commercially available 3,4-dihydroxy-benzaldehyde 26 (10 g, 80 mmol) was dissolved in DMF (50 mL). Join K 2 CO 3 (10 g), and the solution was cooled in an ice-water bath. Add CD slowly 3 I (99 atomic % D, Cambridge Isotopes; 12.4 g, 84 mmol), and the reaction was stirred overnight at room temperature. The reaction was diluted with EtOAc (200 mL) and filtered through a pad of celite. The filtrate was concentrated to give a dark oil. EtOAc (150 mL) and water (50 mL) were added, and the layers were separated. The aqueous phase was adjusted to pH 6 by slow addition of 1N HCl, and the mixture was extracted with EtOAc (2x100 mL). dry (Na 2 SO 4 ) combined or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com