Method for separating and measuring Apremilast and enantiomer of Apremilast through liquid chromatography
A liquid chromatography and enantiomer technology, applied in the field of separation and determination of apremilast and its corresponding isomers by liquid chromatography, can solve the problems of small separation, low sensitivity, low column efficiency, etc. Accurate separation and detection, simple determination method, and the effect of quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
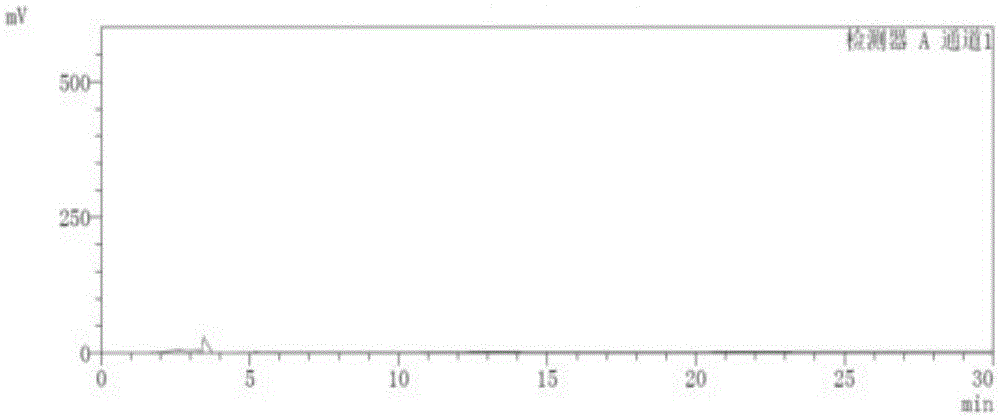
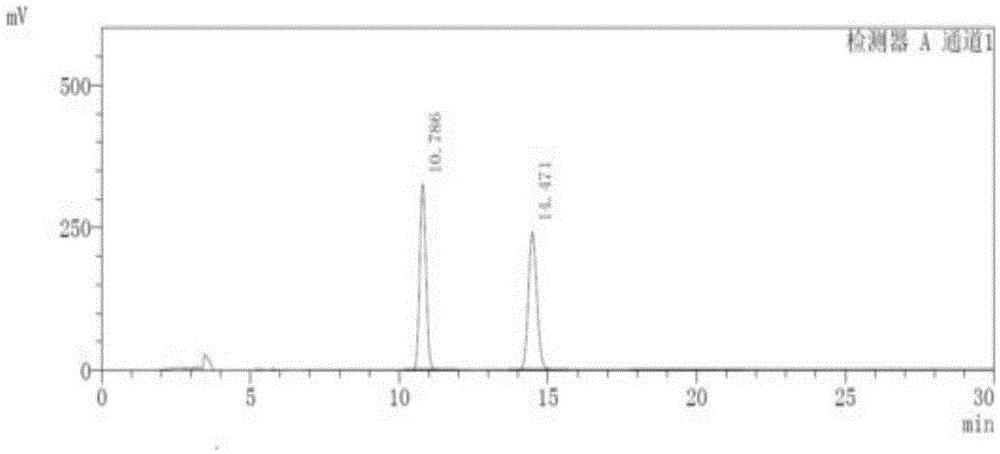
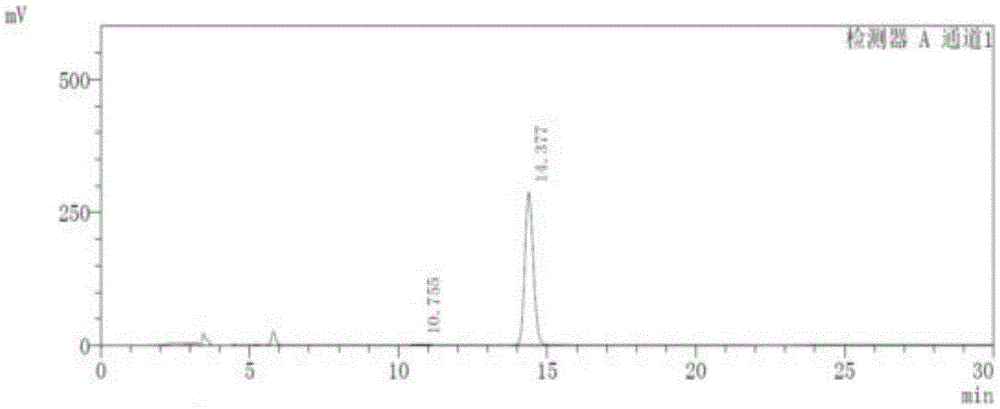
[0036] 1. Instruments and conditions
[0037] Instrument: Shimadzu LC-15C High Performance Liquid Chromatograph
[0038] Chromatographic column: ChiralPakID (5um, 250×4.6mm)
[0039] Mobile phase: methyl tert-butyl ether-ethanol-diethylamine=91:9:0.1
[0040] Detection wavelength: 230nm
[0041] Flow rate: 1.0ml / min
[0042] Column temperature: 30°C
[0043] Injection volume: 10ul
[0044] The sample injection of the present invention is manual sample injection.
[0045] 2. Experimental steps
[0046] Take 5 mg of Apremilast reference substance, accurately weigh it, place it in a 10ml volumetric flask, add an appropriate amount of absolute ethanol, and heat it in a water bath at a temperature of 45°C for an appropriate amount of time to dissolve it; dilute and set the volume to The solution containing 0.5mg in every 1ml is used as the reference substance solution; take 10mg of Apremilast, accurately weigh it, place it in a 10ml volumetric flask, add an appropriate amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com