Separation detection method of apremilast and apremilast enantiomer
An enantiomer and detection method technology, applied in the field of separation and detection of apremilast and its enantiomers, can solve the problems of unpredictable separation effect, failure to meet the minimum resolution requirements, etc., and achieve accuracy High, specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Patent 201410765717.5 method
[0043] Instruments and Conditions
[0044] High performance liquid chromatography: Agilent 1260
[0045] Chromatographic column: AD-H (ChiralPak 250*4.6mm, 5μm)
[0046] Mobile phase: n-hexane-isopropanol-diethylamine-trifluoroacetic acid=80:20:0.2:0.5
[0047] Flow rate: 1.0mL / min
[0048] Detection wavelength: 240nm
[0049] Column temperature: 40°C
[0050] Injection volume: 10μL
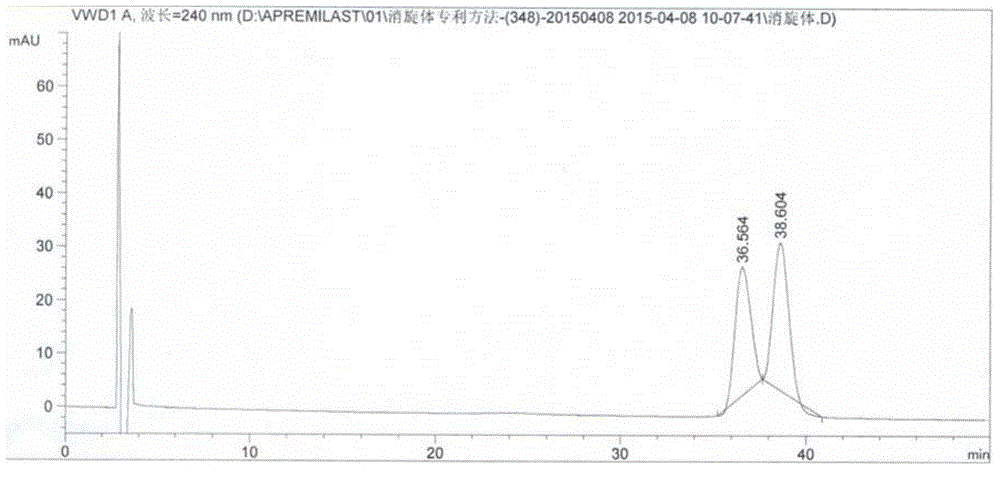
[0051] Take an appropriate amount of Apremilast racemate (batch number: 20140708, self-made by Yabao Pharmaceutical Group Co., Ltd.), add a certain amount of absolute ethanol, dissolve it by ultrasonic waves, and prepare a drug containing 0.1 mg of racemate per 1 mL. solution. Carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, the result sees attached figure 1 . according to figure 1 It can be seen from the results that the separation degree of the two is 1....
Embodiment 2
[0053] Instruments and Conditions
[0054] High performance liquid chromatography: Agilent 1260
[0055] Chromatographic column: AD-H (ChiralPak 250*4.6mm, 5μm)
[0056] Mobile phase: n-hexane-absolute ethanol-diethylamine=50:50:0.1
[0057] Flow rate: 1.0mL / min
[0058] Detection wavelength: 240nm
[0059] Column temperature: 25°C
[0060] Injection volume: 10μL
[0061] Experimental procedure
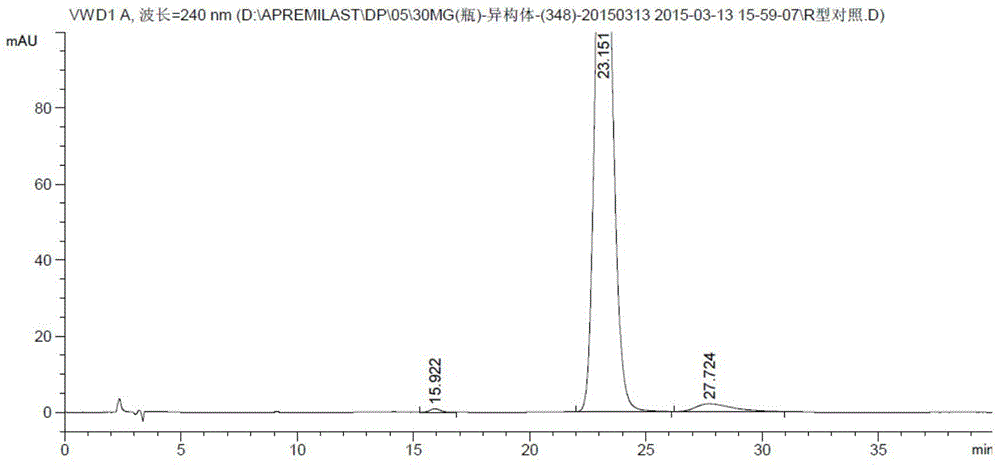
[0062] Take apremilast R-isomer (batch number: 20140719, ee: 99.12%, made by Yabao Pharmaceutical Group Co., Ltd.), racemate (batch number: 20140708, made by Yabao Pharmaceutical Group Co., Ltd.) and Apremilast samples (batch number: 20140829, ee: 98.64%, self-produced by Yabao Pharmaceutical Group Co., Ltd.) are each appropriate amount, respectively, add a certain amount of absolute ethanol, dissolve it by ultrasonic, and make it contain about Premilast R-isomer 0.2mg positioning solution, racemate 0.1mg solution and apremilast 0.5mg sample solution. Carry out high performanc...
Embodiment 3
[0064] Instruments and Conditions
[0065] High performance liquid chromatography: Agilent 1260
[0066] Chromatographic column: AD-H (ChiralPak 250*4.6mm, 5μm)
[0067] Mobile phase: n-hexane-absolute ethanol-diethylamine=50:50:0.1
[0068] Flow rate: 1.0mL / min
[0069] Detection wavelength: 240nm
[0070] Column temperature: 20°C
[0071] Injection volume: 10μL
[0072] Experimental procedure
[0073] Take an appropriate amount of apremilast racemate, add a certain amount of absolute ethanol, dissolve it by ultrasonication, and make a solution containing about 0.1 mg of racemate per 1 mL. Carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, the result sees attached Figure 6 . Depend on Figure 5 It can be known that Apremilil and its enantiomers can be completely separated under this condition, the peak elution time of Apremilil is 31.760min, and the peak elution time of its enantiomers is 27.573min (relative ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com