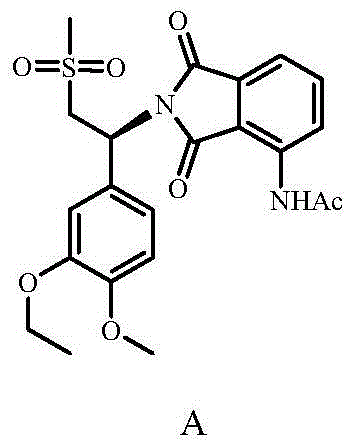
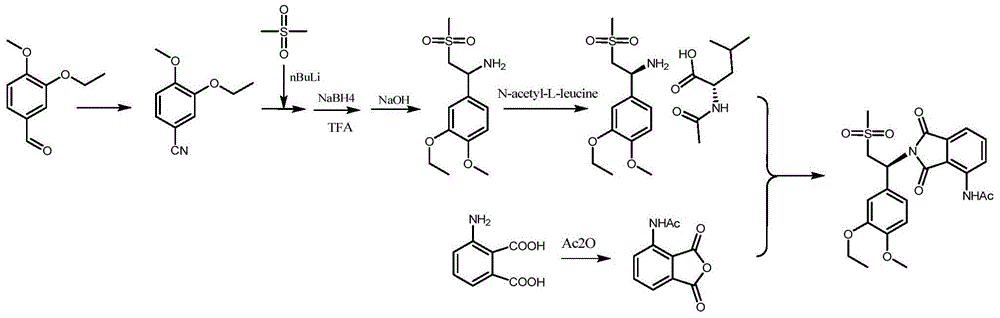
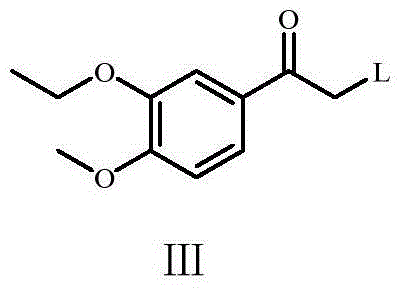
Preparation method of Apremilast and intermediate
A compound and reaction technology, applied in the field of medicine and chemical industry, can solve problems such as inconvenient operation, low yield of 3-acetamidophthalic anhydride, great danger, etc., to facilitate the operation process, reduce production costs, and improve safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 formula II compound
[0045]
[0046] Add 0.25mol of the compound of formula I, 400mL of DMF, 0.25mol of potassium carbonate and 0.30mol of 1-bromoethane into a 1L four-neck flask, and react at 25°C for 4h. The reaction solution was poured into 600 mL of water, stirred, extracted with EA (100 mL×3), the organic phases were combined, washed with saturated brine (100 mL×1), dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain a white solid . Slurry with 100 mL of n-hexane for 10 min, filter, and vacuum-dry the filter cake at 40° C. for 5 h to obtain 42 g of white solid with a yield of 86.5%.
[0047] 1 HNMR (400MHz, DMSO) δ: 7.62(dd, J=8.4, 2.0Hz, 1H), 7.43(d, J=1.9Hz, 1H), 7.07(d, J=8.4Hz, 1H), 4.07(q, J=7.0Hz, 2H), 3.85(s, 3H), 2.53(s, 3H), 1.35(t, J=7.0Hz, 3H).
Embodiment 2
[0048] Synthesis of embodiment 2 formula Ⅲ-1 compound
[0049]
[0050] Add 25mmol of the compound of formula II, 25mmol of phenyltrimethylammonium tribromide, 10mL of tetrahydrofuran and 10mL of methanol into the reaction flask, react at 20°C for 1h, evaporate the solvent under reduced pressure, add 50mL of water and 50mL of EA to the residue, extract and separate , extracted with EA (50mL×2), combined the organic phases, washed once with an appropriate amount of 2M aqueous sodium bicarbonate solution and brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and the residue was vacuum-dried at 40°C for 5h , to obtain 6.0 g of white solid with a yield of 88.2%.
[0051] 1 HNMR (400MHz, CDCl 3 )δ: 7.62(dd, J=8.4, 2.0Hz, 1H), 7.56(d, J=2.0Hz, 1H), 6.93(d, J=8.4Hz, 1H), 4.42(s, 2H), 4.19( q,J=7.0Hz, 2H), 3.97(s,3H), 1.51(t,J=7.0Hz, 3H).
Embodiment 3
[0052] Synthesis of embodiment 3 formula Ⅲ-2 compound
[0053]
[0054] Add 50mmol of the compound of formula II, 100mL of methanol and 25mmol of p-toluenesulfonic acid into a 250mL four-neck flask. The ice-water bath is lower than 5°C. Dichlorohydantoin is added in 3 batches, totaling 37.5mmol, and the temperature is controlled below 10°C. After the addition was completed, the temperature was naturally raised to 25°C, the system became clear, and a white solid was precipitated after reacting for 15 hours. The solvent was evaporated under reduced pressure, and the residue was added with 100 mL each of water and EA, shaken to separate the liquid, and extracted with EA (50 mL×3). The organic phases were combined, washed with saturated brine (100 mL×1), dried over anhydrous magnesium sulfate, filtered, and the filtrate was distilled off the solvent under reduced pressure to obtain a light yellow solid. Wash with 2 mL of EA, filter, and vacuum-dry the filter cake at 40° C. for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com