Topical compositions of apremilast
A composition, topical medicine technology, applied in the field of treating skin diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Embodiment 1 (Apremilast topical foamable composition)
[0150] Table 1
[0151]
[0152]
[0153] Process for preparing apremilast topical foamable formulation
[0154] Apremilast was dissolved in isosorbide dimethyl ether and / or diethylene glycol monoethyl ether (Transcutol) to obtain a drug solution. Polyethylene glycol (15)-hydroxystearate, ceteareth 20, cetyl alcohol, PEG-400, glyceryl monocaprylate, polysorbate and phenoxyethanol according to Table 1 Melt and mix to make the oil phase. The previously prepared drug solution was added dropwise to the oil phase and stirred to obtain a mixture. PVP-K30 in Example 1a was added to water to prepare an aqueous phase, and water was used as the aqueous phase in Example 1b. Add the aqueous phase to the prepared mixture and stir slowly to obtain a microemulsion. The microemulsion thus prepared was filled in an aluminum can, and a propellant (deodorized liquefied petroleum gas (LPG)) was added in Example 1b to obtain ...
Embodiment 2
[0155] Embodiment 2 (Apremilast topical foamable composition)
[0156] Table 2
[0157]
[0158] Process for preparing apremilast topical foamable formulation
[0159] Apremilast was dissolved in isosorbide dimethyl ether and / or diethylene glycol monoethyl ether (Transcutol) to obtain a drug solution. Melt and mix other excipients such as polyethylene glycol (15)-hydroxystearate, ceteareth 20, cetyl alcohol, PEG-400, glyceryl monocaprylate and phenoxyethanol to prepare the oil Mutually. The previously prepared drug solution was added dropwise to the oil phase and stirred to obtain a homogeneous mixture. Add PVP-K30 to water to prepare an aqueous phase. Add the aqueous phase to the prepared homogeneous mixture and stir slowly to obtain a microemulsion. The microemulsion thus prepared was filled in an aluminum can, and finally a propellant (deodorized liquefied petroleum gas (LPG)) was added to obtain a topical microemulsion foam of Apremilast.
Embodiment 3
[0160] Embodiment 3 (Apremilast topical foamable composition)
[0161] table 3
[0162]
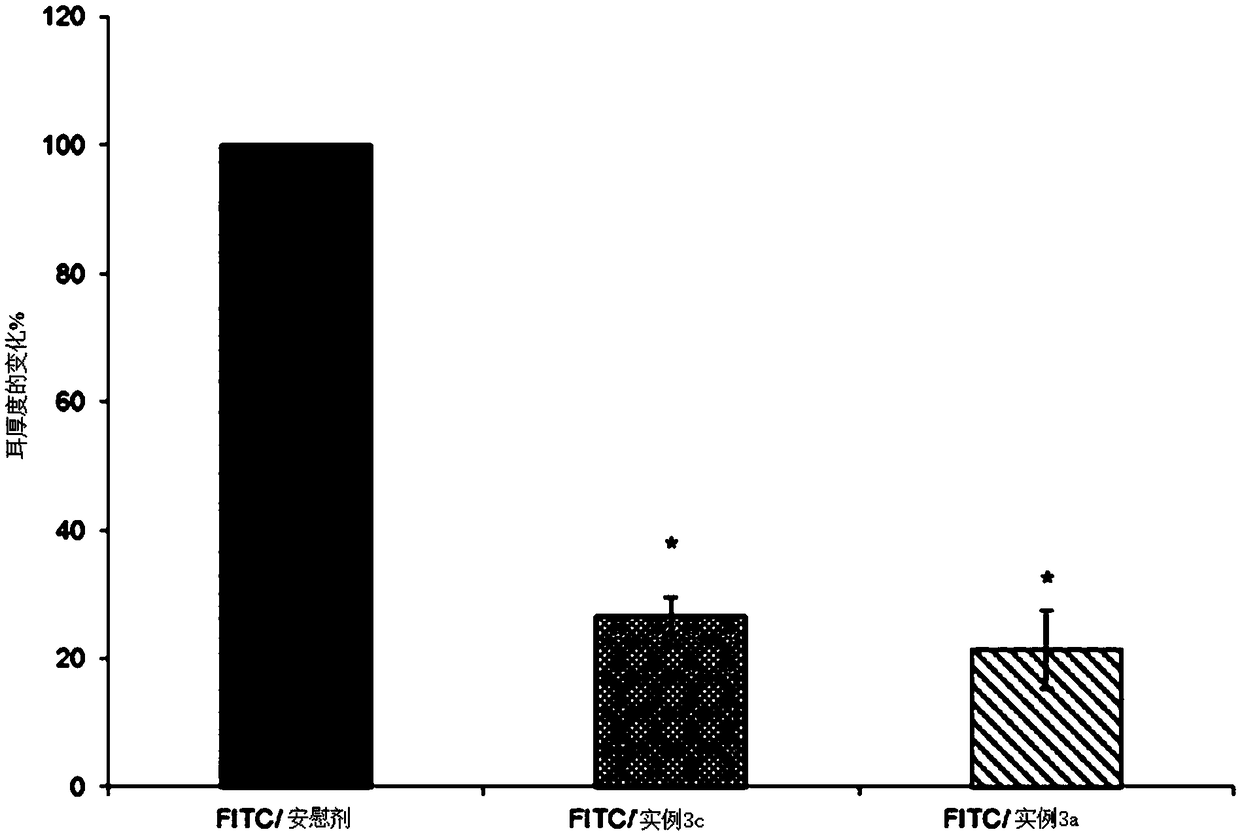
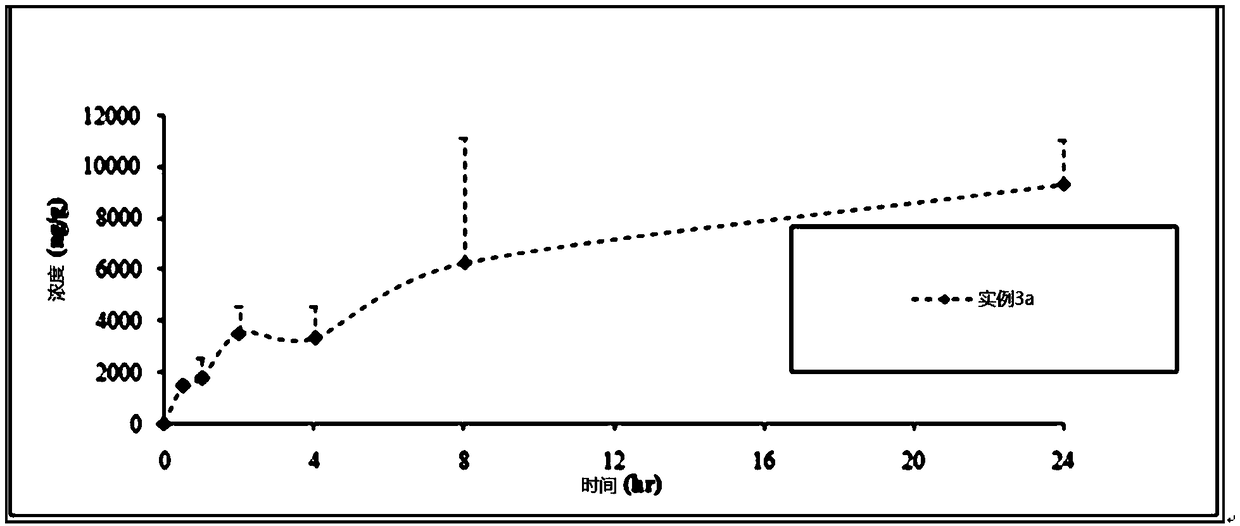
[0163] The compositions of Examples 3a-3d were prepared according to the method described in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com