Therapeutic topical compositions of apremilast
A composition and topical application technology, applied in the field of topical therapeutic composition of Apremilast, can solve the problem that oral tablet composition is not suitable for swallowing difficulties or gastrointestinal side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
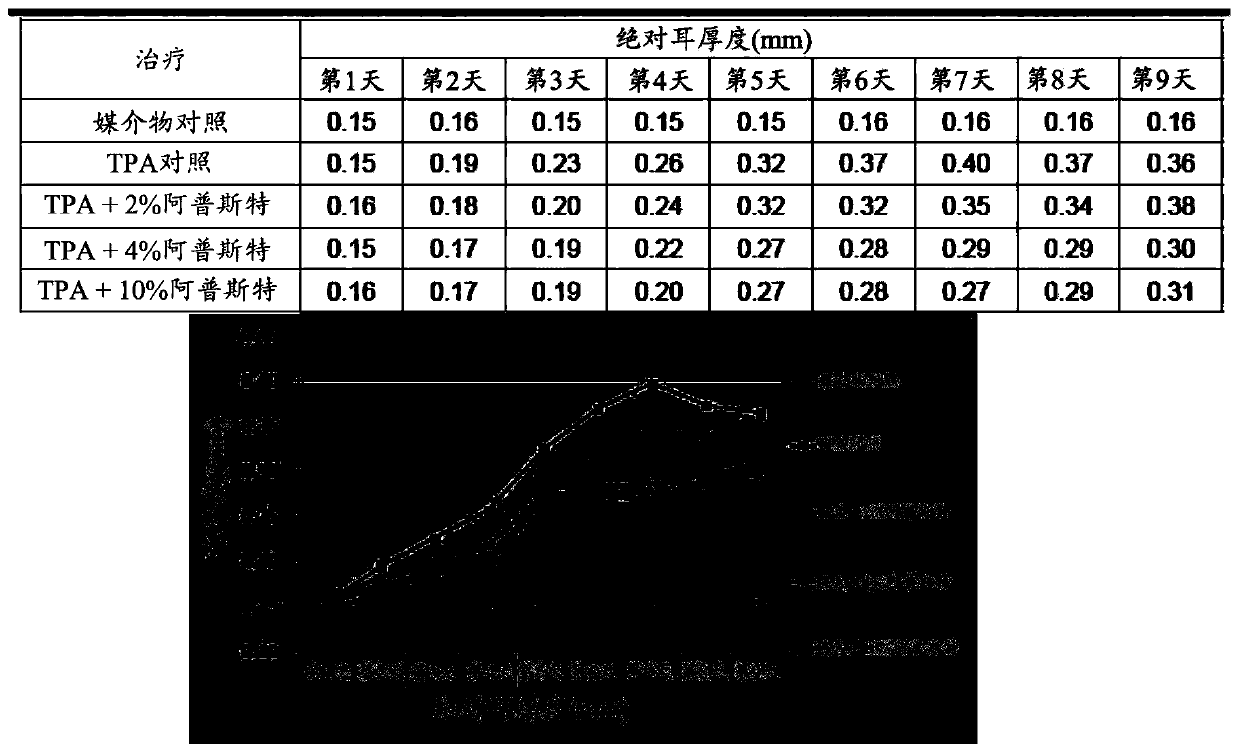
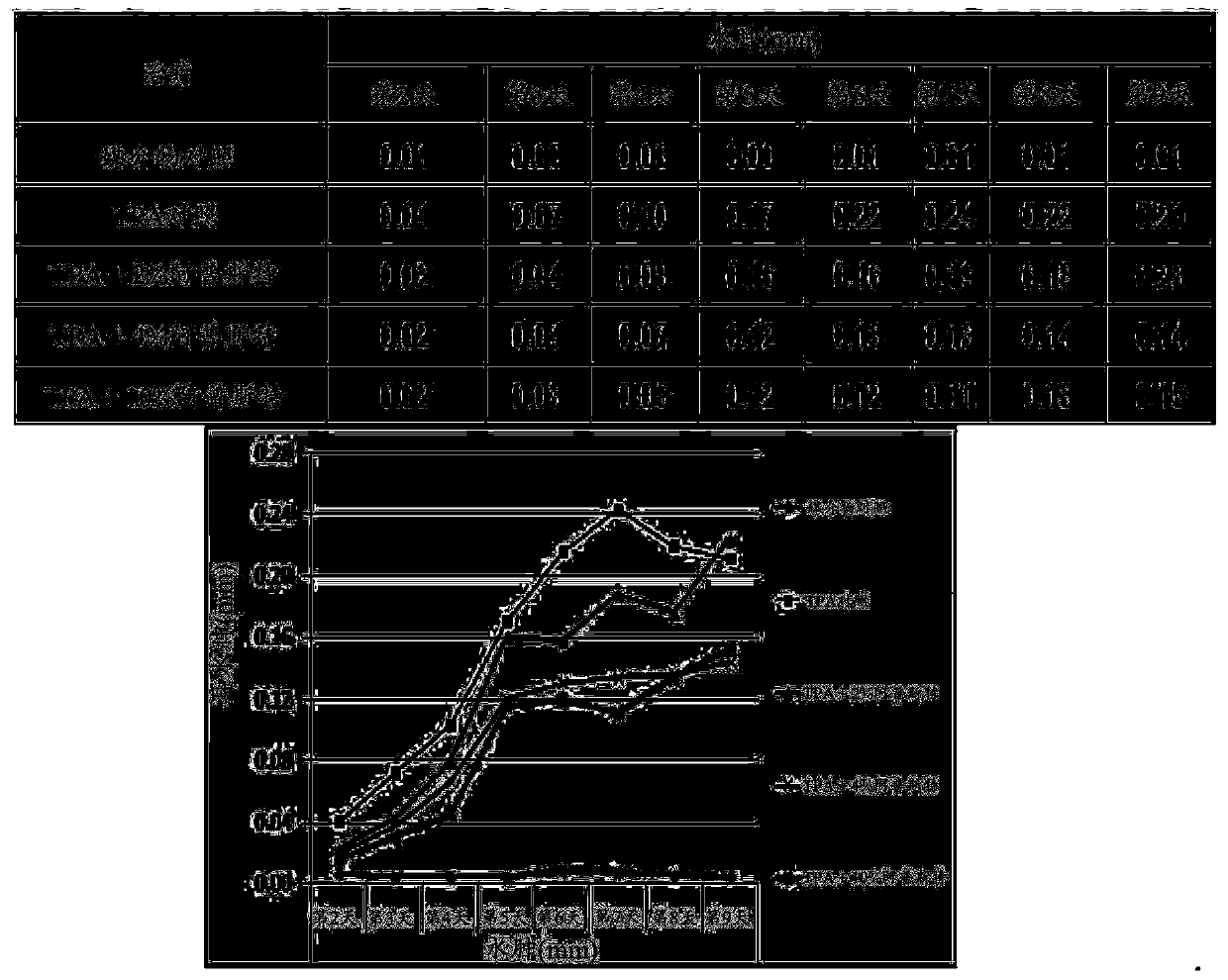
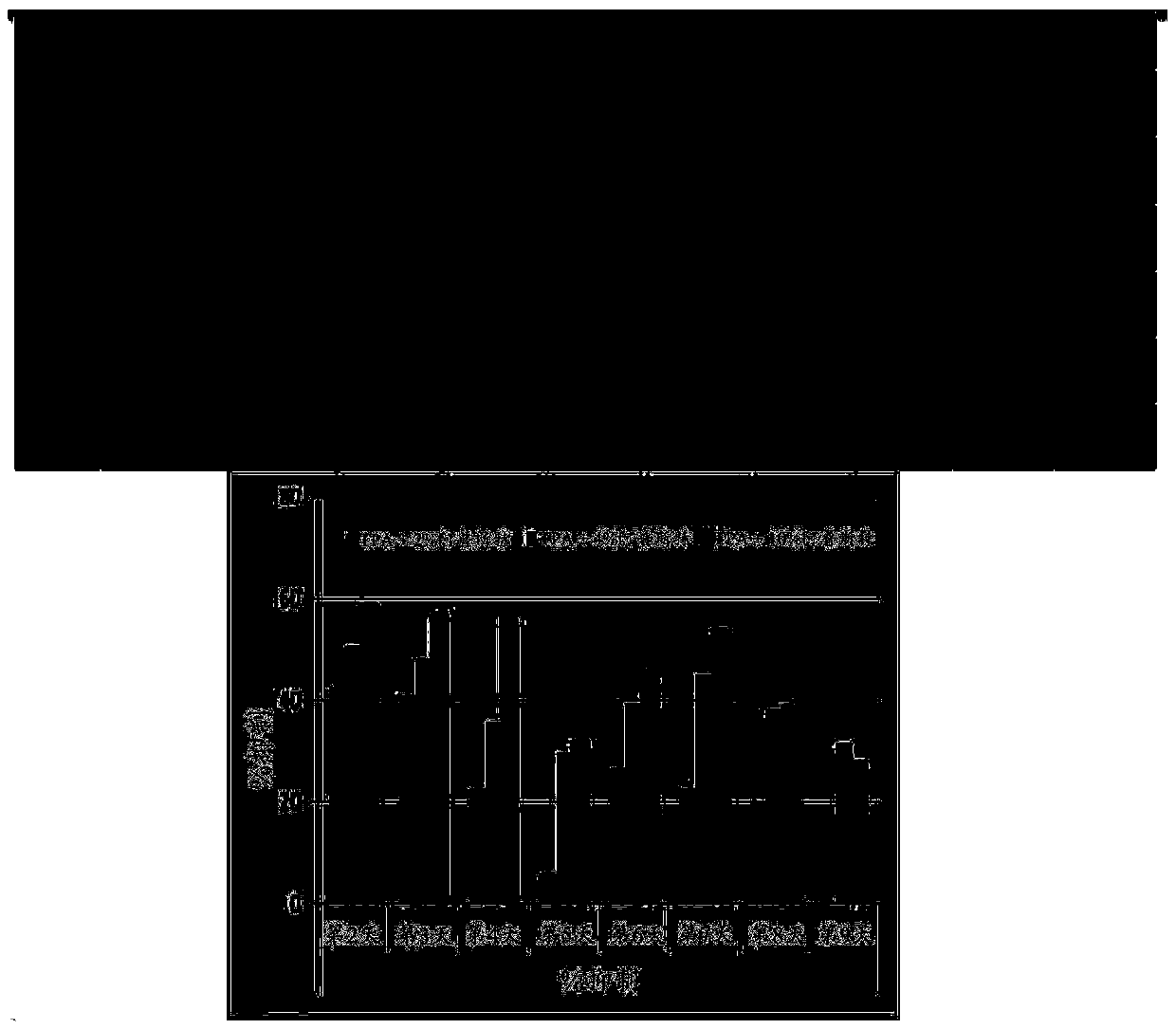
Image
Examples
Embodiment 1
[0082] Example 1: Topical Composition of Apremilast
[0083]
[0084] program:
[0085] a) Dissolve apremilast in a portion of ethanol while stirring.
[0086] b) Add isopropyl myristate and undecylenic acid to the solution of step (a) while stirring.
[0087] c) Add the remaining amount of ethanol to the solution of step (b) and mix.
Embodiment 2
[0088] Example 2: Topical Composition of Apremilast
[0089]
[0090] program:
[0091] a) Dissolve apremilast in a portion of ethanol while stirring.
[0092] b) Dissolve iodide and sodium thiosulfate in water.
[0093] c) Adding the solution obtained in step (b) to the solution of step (a) while stirring.
[0094] d) Make up the volume to the required amount by adding ethanol and water to the solution of step (c) and mixing.
Embodiment 3
[0095] Example 3: Topical Composition of Apremilast
[0096]
[0097] program:
[0098] a) Dissolve apremilast in a mixture of isopropyl myristate, mineral oil and carbitol with stirring.
[0099] b) Dissolve the acrylic acid polymer in ethanol.
[0100] c) Adding the solution obtained in step (b) to the solution of step (a) while stirring.
[0101] d) Make up the volume to the required amount by adding ethanol and mineral oil to the solution of step (c) and mixing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com