Apremilast gel for injection to articular cavity and preparation method of apremilast gel
A special gel and gel technology, applied in the field of medicine, can solve problems such as increased risk of depression and weight loss, achieve the effects of reducing systemic adverse reactions, reducing frequent drug administration, and expanding clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
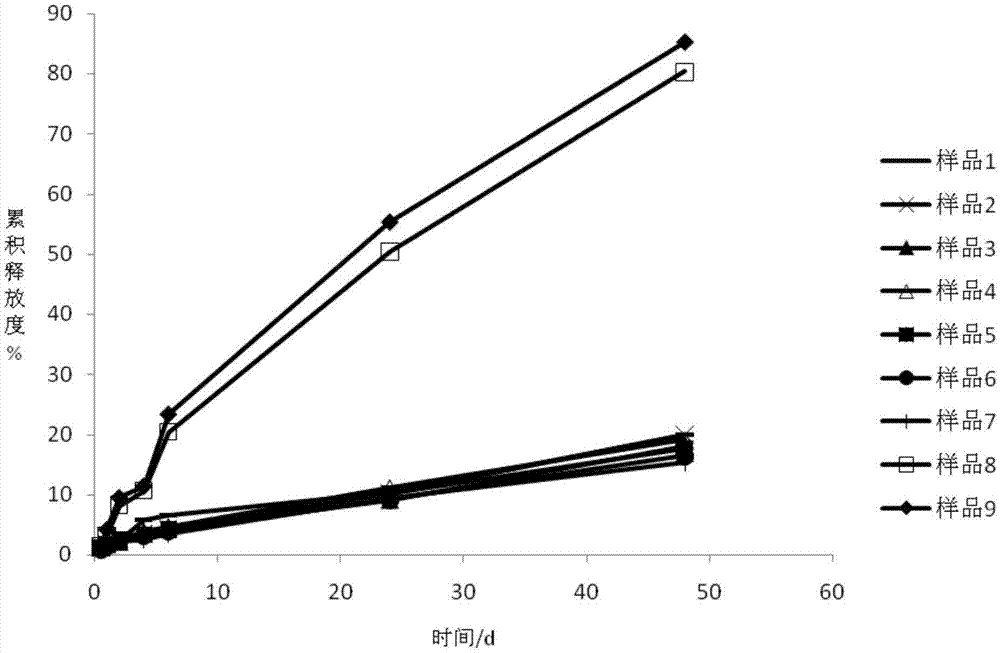
[0026] Below 30°C, add the prescribed amount of poloxamer 407, poloxamer 188 and glycerin into purified water, and stir to form a poloxamer sol solution. After adding Apremilast, use a 12000rpm high-speed shear emulsifier to mix for 1h, and stir at 50rpm for 5h to dissolve. Centrifuge at 10,000rpm for 10 minutes to take the supernatant, and use a 0.22μm membrane to filter and sterilize in a sterile operating room to obtain this product (sample 1).
Embodiment 2
[0028]
[0029] Below 30°C, add the prescribed amount of poloxamer 407 and glycerin into purified water, and stir to form a poloxamer sol solution. After adding Apremilast, use a 12000rpm high-speed shear emulsifier to mix for 1h, and stir at 50rpm for 5h to dissolve. Centrifuge at 10,000rpm for 10 minutes to get the supernatant, filter and sterilize with a 0.22μm membrane in a sterile operating room to obtain this product (sample 2).
Embodiment 3
[0031]
[0032] Below 30°C, add the prescribed amount of poloxamer 407, poloxamer 188 and glycerin into purified water, and stir to form a poloxamer sol solution. After adding Apremilast, use a 12000rpm high-speed shear emulsifier to mix for 1h, and stir at 50rpm for 5h to dissolve. Centrifuge at 10,000rpm for 10 minutes to take the supernatant, and use a 0.22μm membrane to filter and sterilize in a sterile operating room to obtain this product (sample 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com