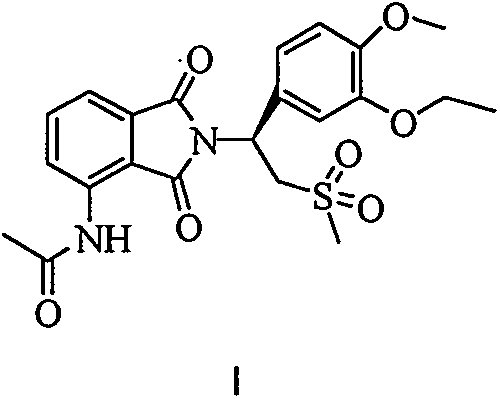
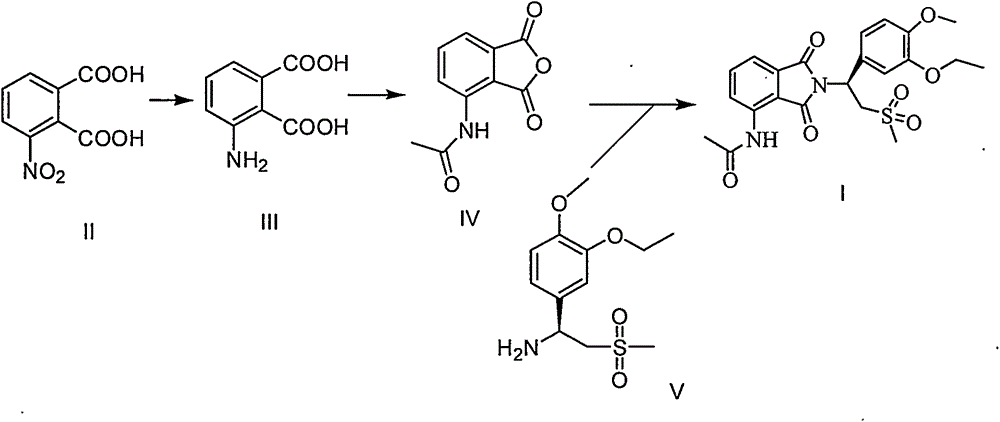
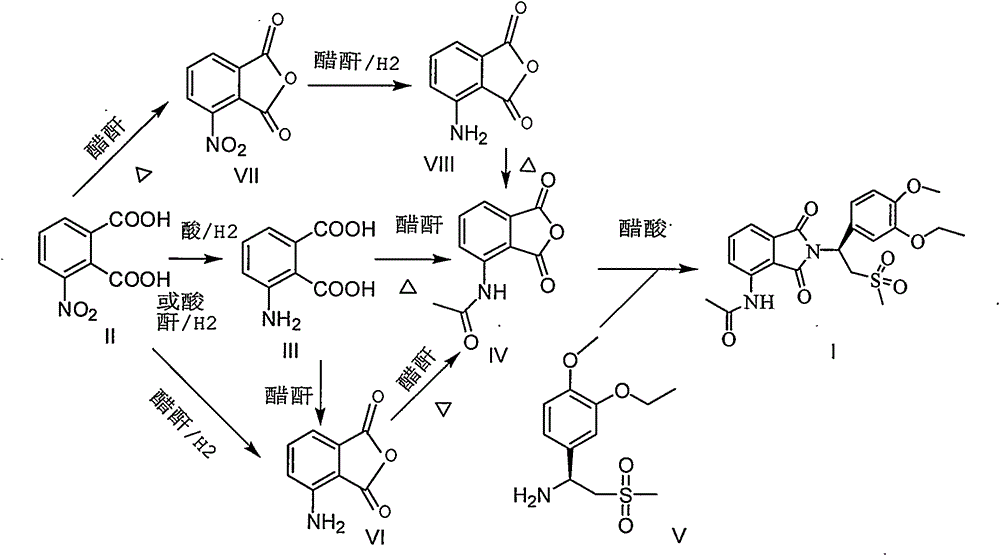
Industrial method for preparing apremilast and intermediate thereof
A technology for intermediates and compounds, applied in the field of medicinal chemistry, can solve the problems of high cost, low yield, and difficulty in industrialization, and achieve the effects of low cost, high yield and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of 3-aminophthalic acid
[0027] 2-Nitrophthalic acid (1.0kg), glacial acetic acid (8.5L), and 10% Pd / C (30g) were added to the autoclave in turn, replaced with nitrogen twice, and hydrogen was introduced to 1MPa, and the pressure was maintained at room temperature. React under pressure until no more hydrogen is absorbed, vent, blow with nitrogen, cool, and filter to obtain 920 g of a product containing palladium on carbon, with a solvent content of about 15%.
Embodiment 2
[0028] Embodiment 2: Preparation of 3-acetamidophthalic anhydride
[0029] Transfer the undried product obtained in Example 1 into a 5L three-necked flask, add acetic anhydride (4.5L), heat to reflux for 1-2 hours, filter while hot, wash with glacial acetic acid, and then cool the filtrate to 0-5°C. Stir until the product is fully separated, filter, and wash with glacial acetic acid to obtain 827 g of yellow needle-like crystals, containing about 12% solvent, 728 g after drying, 75% yield in two steps, and 99.8% by HPLC.
Embodiment 3
[0030] Embodiment 3: the preparation of Apremilast
[0031] The above undried product (728g after drying), glacial acetic acid (4.2L), (S)-1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonyl)ethyl Amine N-acetyl-L-leucine salt (1570g, ee99.3%) was added to the reaction flask in turn, and heated to reflux for 1 hour. After TLC detected that the raw materials disappeared, the reaction was terminated, glacial acetic acid was recovered under reduced pressure, and dichloromethane was added. Dissolve in 10L, successively wash with 5L of water, 5L of saturated aqueous sodium bicarbonate solution, 5L of saturated sodium chloride, dry over anhydrous magnesium sulfate, concentrate to dryness under reduced pressure, add 10L of absolute ethanol, reflux for 30 minutes, filter, wash with ethanol, After drying at 60°C, 1584 g of the product was obtained, with a yield of 97%, an HPLC above 99.8%, and an ee of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com