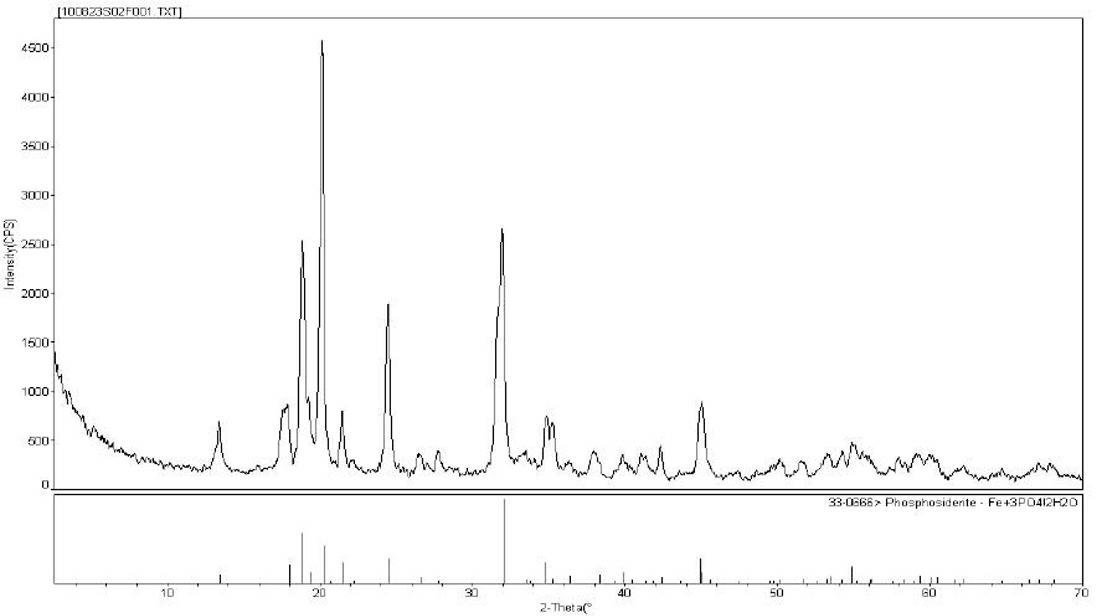
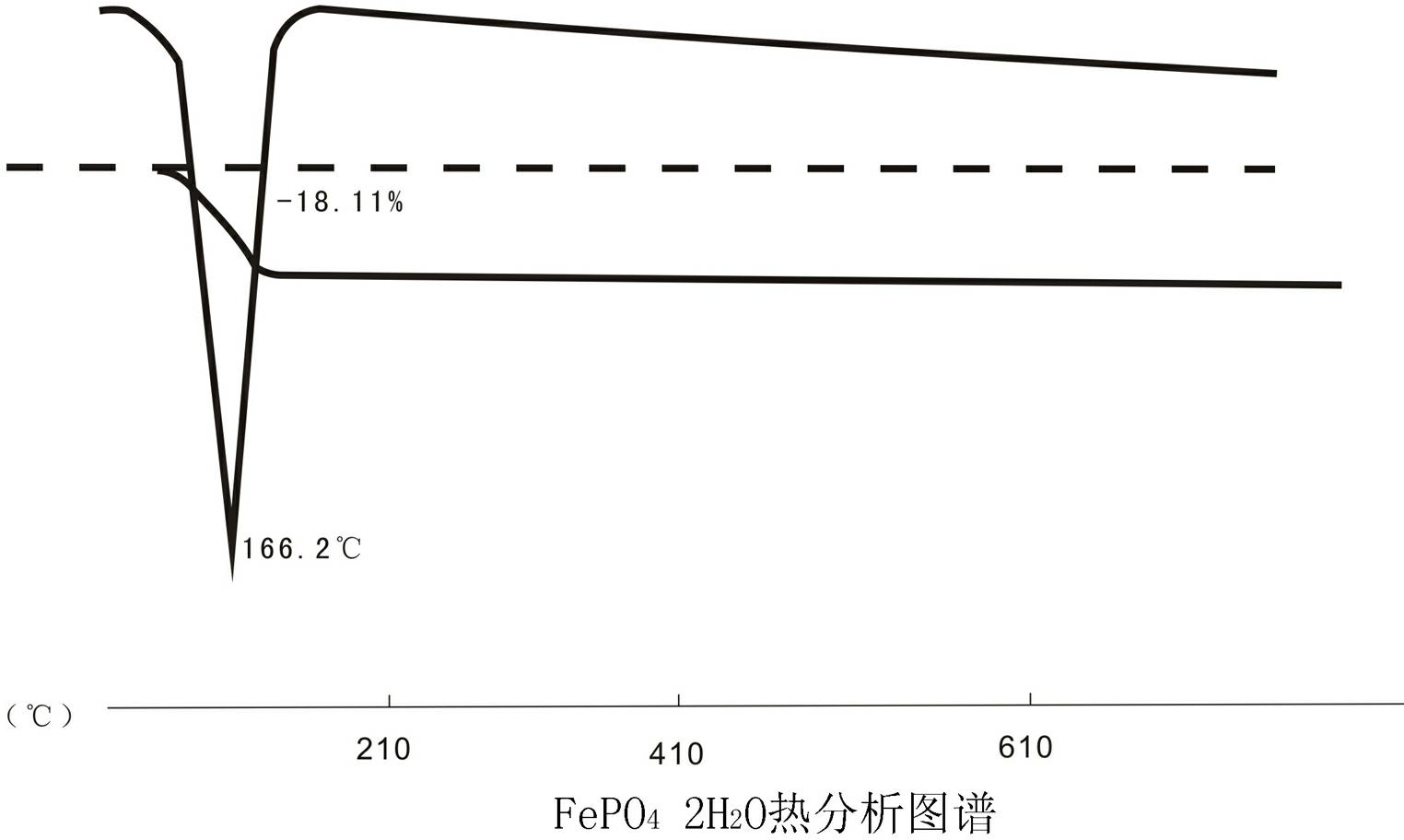
Method for preparing spherical iron phosphate for lithium iron phosphate cell material
A lithium iron phosphate battery and manufacturing method technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problem of poor rate discharge performance, low LFP gram capacity, and product quality that does not meet the requirements of battery-grade products, etc. problem, to achieve a highly dispersed and fluid effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Weigh 1000KG ferrous sulfate crystals, add water to dissolve and filter, transfer to a clean 5000L reactor, fully
[0031] Stir and raise the temperature to about 50 degrees, add about 5KG of acid to stabilize the pH value at about 1.0~2.0, then add 200~350KG of industrial hydrogen peroxide and stir for about 60 minutes to make the solution turn brownish red. Then slowly add industrial ammonia water to adjust the pH value to about 4.5~6, so that iron can form ferric hydroxide precipitation. After continuing to raise the temperature to 80-95 degrees, filter under high pressure (about greater than 10KG pressure) while it is hot, and fully wash the ferric hydroxide filter cake with pure water at about 80 degrees until the pH value of the filter cake is stable at 6-7. The filter cake is transferred to the PP storage tank for use.
[0032] 2. Prepare a 5-18% dilute phosphoric acid solution, transfer it to a 5000L reactor, start stirring at a speed of 80r / min, and raise t...
Embodiment 1
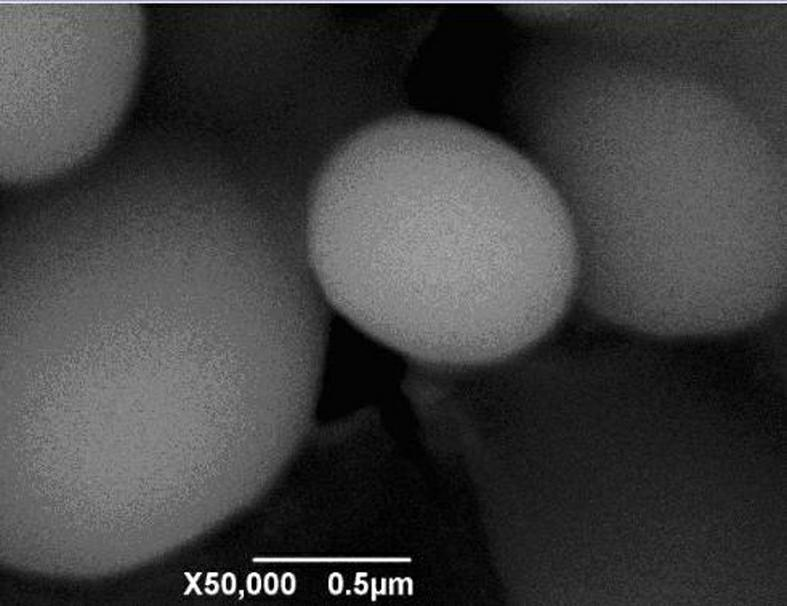
[0041] The ferric phosphate SEM figure that embodiment 1 obtains is as follows image 3 As shown, the laser particle size distribution map of the finished product is as follows Figure 4 shown.
Embodiment 2
[0043] 1. Weigh 800KG iron oxide and 300KG iron flakes, add 30% by-product hydrochloric acid or sulfuric acid 2000KG and heat up to 80~95 degrees to dissolve the iron source. The reaction is accompanied by metathesis reaction, redox reaction and replacement reaction. It can be filtered when it is green, transfer the filtrate to a clean 5000L reactor, stir well and raise the temperature to about 50 degrees, add about 5KG of acid to stabilize the pH value at about 1.0~2.0, and then add industrial hydrogen peroxide 200~ 350KG was fully stirred for about 60 minutes to make the solution turn brownish red. Then slowly add industrial ammonia water to adjust the pH value to about 4.5~6, so that the iron salt forms iron hydroxide precipitation. After continuing to raise the temperature to 80-95 degrees, filter under high pressure (approximately greater than 10KG pressure) while hot, and fully wash the iron hydroxide filter cake with pure water at about 80 degrees until the pH value of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com