Cefepime hydrochloride composition for injection and its preparation method
A composition and technology for injection, applied in the field of medicinal chemistry, can solve problems such as nutritional anemia, dysplasia, and central nervous system obstruction, and achieve the effect of simple preparation process, reasonable composition, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] prescription:
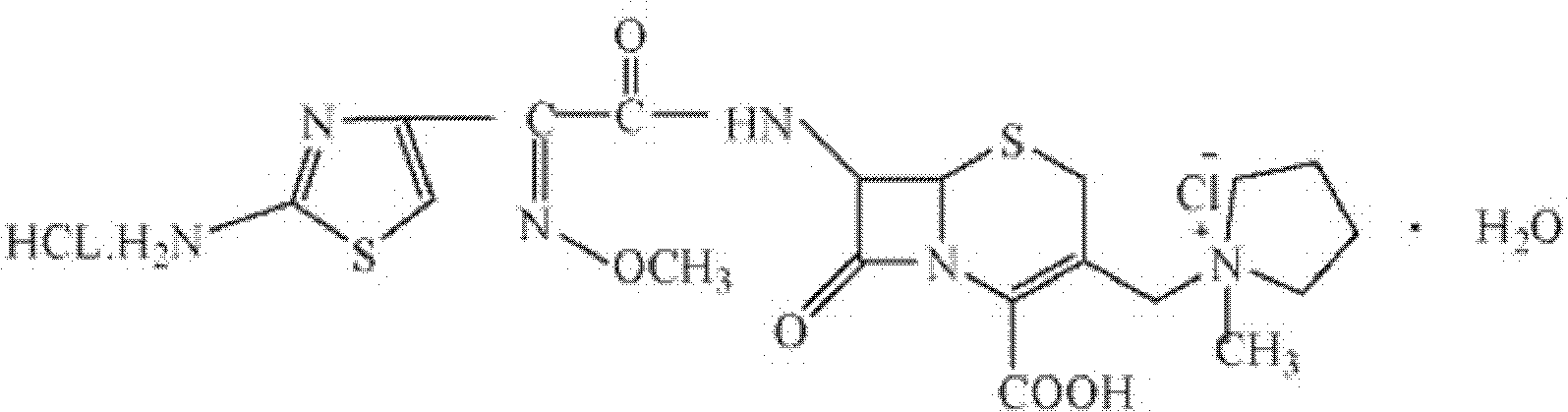
[0045] Cefuroxime hydrochloride (calculated as cefoxime) 1000g
[0046] L-Arginine 710g
[0047] L-Lysine 10g
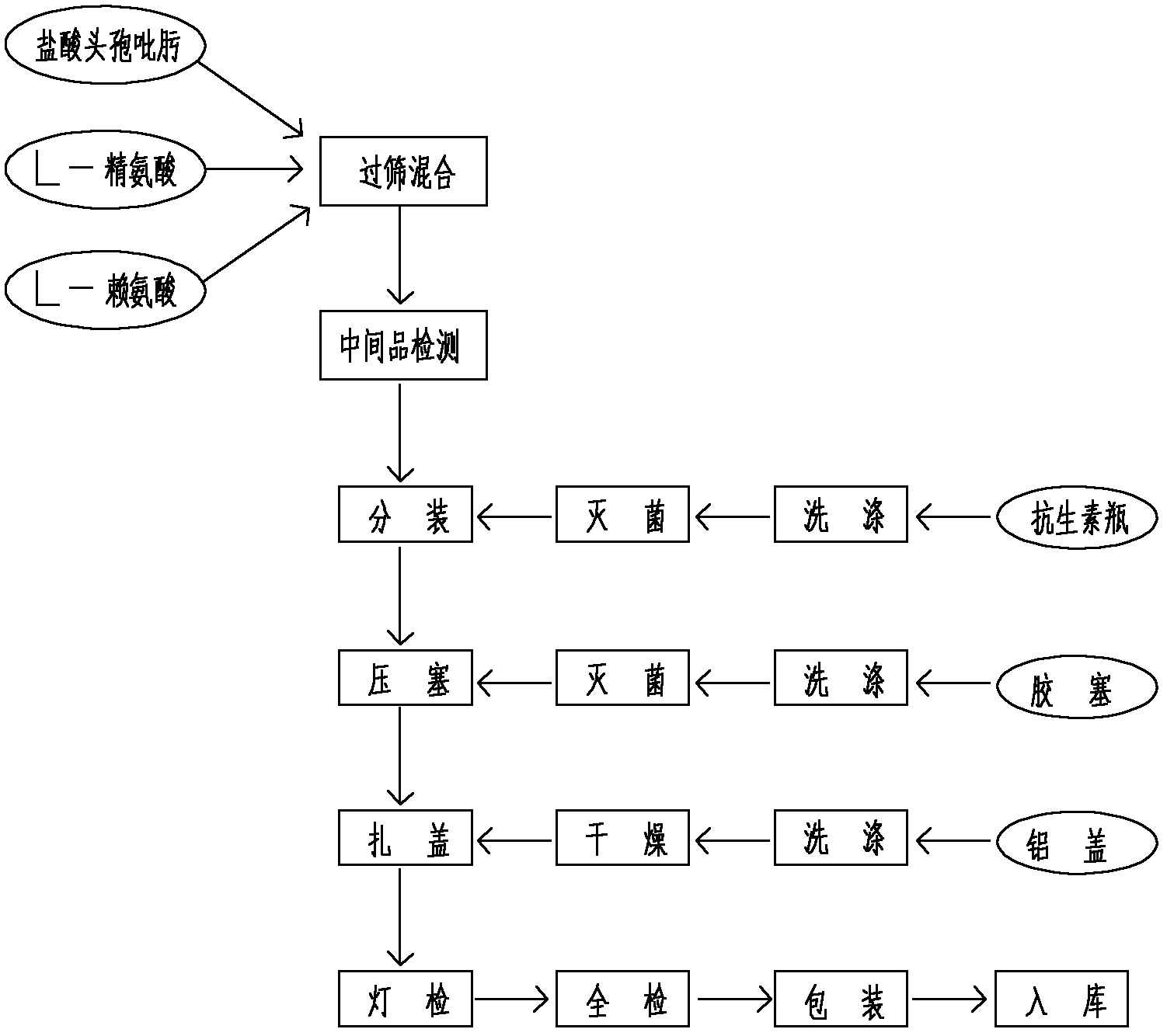
[0048] Preparation process (see attached figure 1 ):
[0049] (1) After the original and auxiliary materials are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0050] (2) In the 100-level clean area, cefoxime hydrochloride, L-arginine and L-lysine were respectively pulverized and passed through an 80-mesh sieve;
[0051] (3) Weigh cefoxime hydrochloride, L-arginine and L-lysine in a 100-level clean area, mix them evenly according to the prescription ratio, and pack them into control bottles after the measured content is qualified, press the stopper, roll the cap, After passing the light inspection and inspection, it can be labeled and packaged.
Embodiment 2
[0053] prescription:
[0054] Cefuroxime hydrochloride (calculated as cefoxime) 1000g
[0055] L-Arginine 710g
[0056] L-Lysine 15g
[0057] Preparation Process:
[0058] (4) After the original and auxiliary materials are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0059] (5) In the 100-level clean area, cefoxime hydrochloride, L-arginine and L-lysine were respectively pulverized and passed through an 80-mesh sieve;
[0060] (6) Weigh cefoxime hydrochloride, L-arginine and L-lysine in a 100-level clean area, mix them evenly according to the prescription ratio, and subpackage them in control bottles after the measured content is qualified. After passing the light inspection and inspection, it can be labeled and packaged.
Embodiment 3
[0062] prescription:
[0063] Cefuroxime hydrochloride (calculated as cefoxime) 1000g
[0064] L-Arginine 710g
[0065] L-Lysine 20g
[0066] Preparation Process:
[0067] (7) After the original and auxiliary materials are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0068] (8) In a 100-level clean area, cefoxime hydrochloride, L-arginine and L-lysine are respectively pulverized and passed through an 80-mesh sieve;
[0069] (9) Take cefoxime hydrochloride, L-arginine and L-lysine in a 100-level clean area, mix them evenly according to the prescription ratio, and pack them in control bottles after the measured content is qualified, press the stopper, roll the cap, After passing the light inspection and inspection, it can be labeled and packaged.
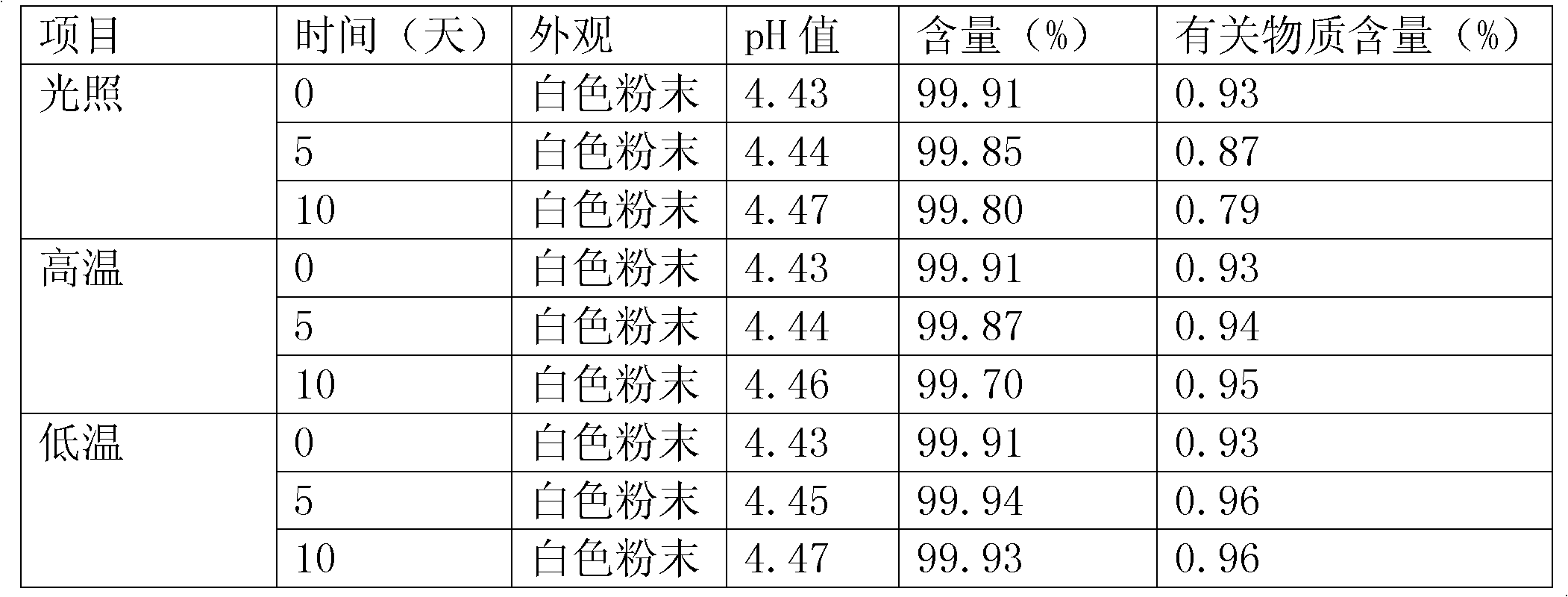
[0070] The beneficial effects of the present invention are described through the following experimental examples and comparative examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com