Synthesis method of cefepime hydrochloride
The technology of a kind of cefepime hydrochloride and synthetic method is applied in the field of synthesis of cefepime hydrochloride to achieve the effects of cost reduction, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
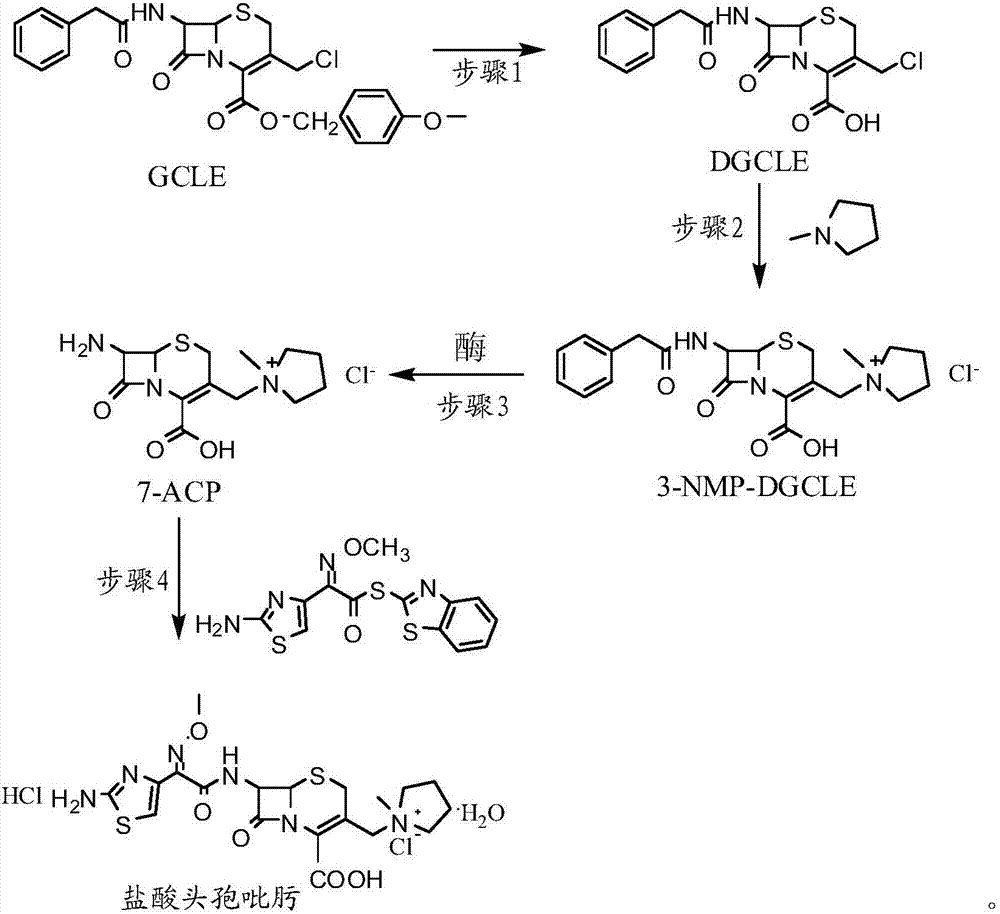
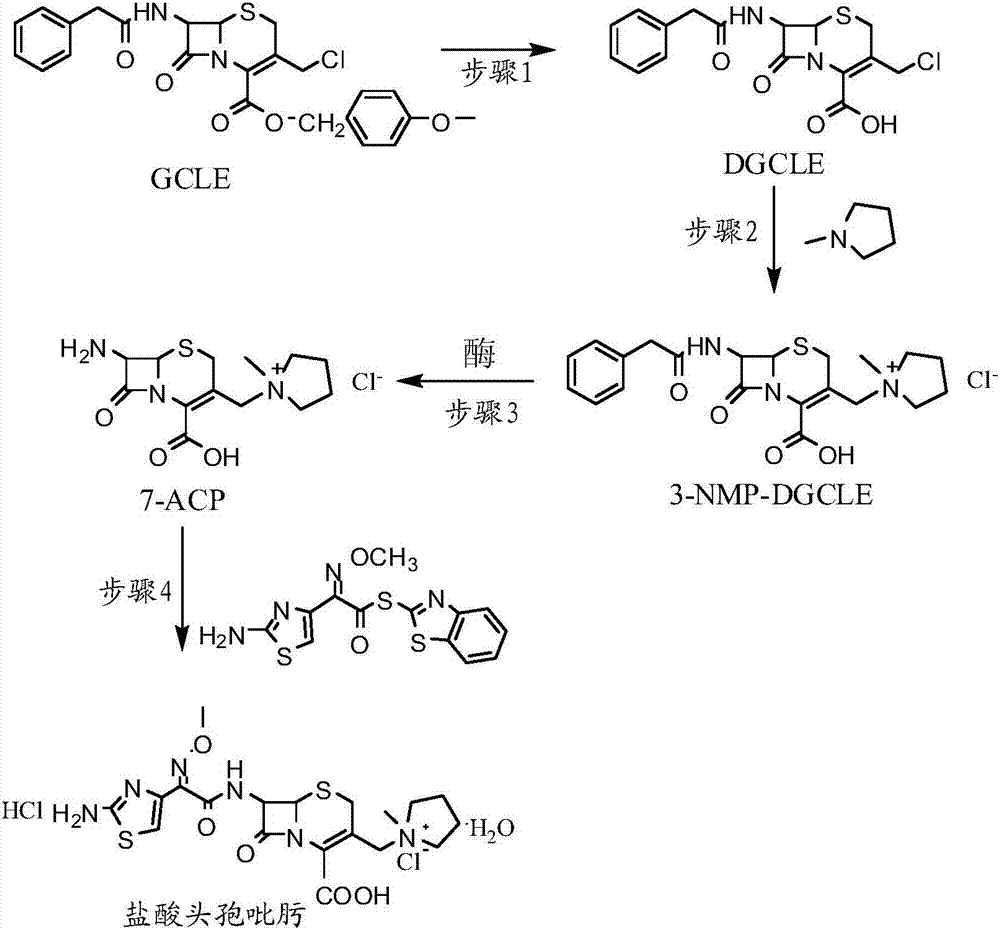
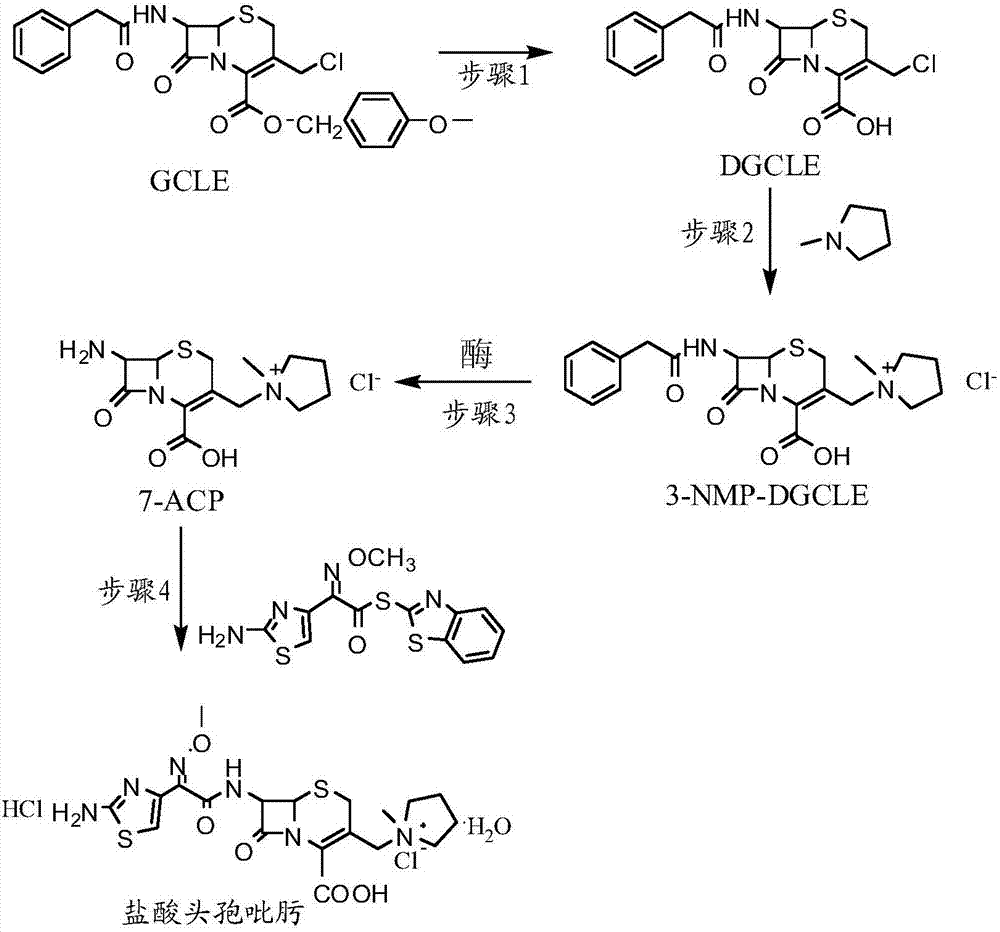
[0032] Step 1: Synthesis of 7-phenylacetamido-3-chloromethyl-3-cephalosporin-4-carboxylic acid (DGCLE)
[0033]Dissolve 20g of GCLE in 50ml of dichloromethane, and after cooling the system to -25°C, add 19.8ml of trifluoroacetic acid (the molar ratio to GCLE is 6.5:1) to the reaction solution; the mixture is stirred and reacted at -25°C for 16 hours; The reaction solution was transferred to a rotary evaporator to distill off 2 / 3 of the solvent under reduced pressure, and 200 ml of petroleum ether was added to the reaction solution to precipitate crystals; dried to obtain 12.9 g of compound I (DGCLE), with a molar yield of 85.6%.
[0034] 7-phenylacetamido-3-chloromethyl-3-cephalosporin-4-carboxylic acid (DGCLE) was analyzed and characterized by NMR.
[0035] NMR data: 1 HNMR (500MHz, CDCl 3 )δ3.52(s,2H,COCH 2 ); 3.71, 3.82 (d, J=7.5Hz, 2H, SCH 2 ); 4.05 (s,2H,ClCH 2 ); 4.19 (ABq, J=13.5Hz, 1H, N + CH 2 ), 4.61 (ABq, J=14.0Hz, 1H, N + CH 2 ), 5.13 (S, 1H, C-6, β-lactam...
Embodiment 2
[0049] Step 1: Synthesis of 7-phenylacetamido-3-chloromethyl-3-cephalosporin-4-carboxylic acid (DGCLE)
[0050] Dissolve 20g of GCLE in 50ml of ethyl acetate, and after cooling the system to -25°C, add 21.3ml of trifluoroacetic acid (the molar ratio to GCLE is 7.0:1) to the reaction solution. The mixture was stirred and reacted at -25°C for 16 hours; the reaction solution was transferred to a rotary evaporator to distill off 2 / 3 of the solvent under reduced pressure, and 200ml of n-hexane was added to the reaction solution to precipitate crystals; dried; to obtain compound I (DGCLE) 12.8 g, molar yield 85.3%.
[0051] 7-phenylacetamido-3-chloromethyl-3-cephalosporin-4-carboxylic acid (DGCLE) was analyzed and characterized by NMR.
[0052] NMR data: 1 HNMR (500MHz, CDCl 3 )δ3.52(s,2H,COCH 2 ); 3.71, 3.82 (d, J=7.5Hz, 2H, SCH 2 ); 4.05 (s,2H,ClCH 2 ); 4.19 (ABq, J=13.5Hz, 1H, N + CH 2 ), 4.61 (ABq, J=14.0Hz, 1H, N + CH 2 ), 5.13 (S, 1H, C-6, β-lactam); 5.42 (dd, J=4.0H...
Embodiment 3
[0066] Step 1: Synthesis of 7-phenylacetamido-3-chloromethyl-3-cephalosporin-4-carboxylic acid (DGCLE)
[0067] Dissolve 20g of GCLE in 50ml of chloroform, and after cooling the system to -30°C, add 14.2ml of formic acid (the molar ratio to GCLE is 7.5:1) to the reaction solution; the mixture is stirred and reacted at -30°C for 16 hours. The reaction solution was transferred to a rotary evaporator to distill off 2 / 3 of the solvent under reduced pressure, and 200 ml of cyclohexane was added to the reaction solution to precipitate crystals; dried to obtain 13.1 g of compound I (DGCLE), with a molar yield of 86.1%.
[0068] 7-phenylacetamido-3-chloromethyl-3-cephalosporin-4-carboxylic acid (DGCLE) was analyzed and characterized by NMR.
[0069] NMR data: 1 HNMR (500MHz, CDCl 3 )δ3.52(s,2H,COCH 2 ); 3.71, 3.82 (d, J=7.5Hz, 2H, SCH 2 ); 4.05 (s,2H,ClCH 2 ); 4.19 (ABq, J=13.5Hz, 1H, N + CH 2 ), 4.61 (ABq, J=14.0Hz, 1H, N + CH 2 ), 5.13 (S, 1H, C-6, β-lactam); 5.42 (dd, J=4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com