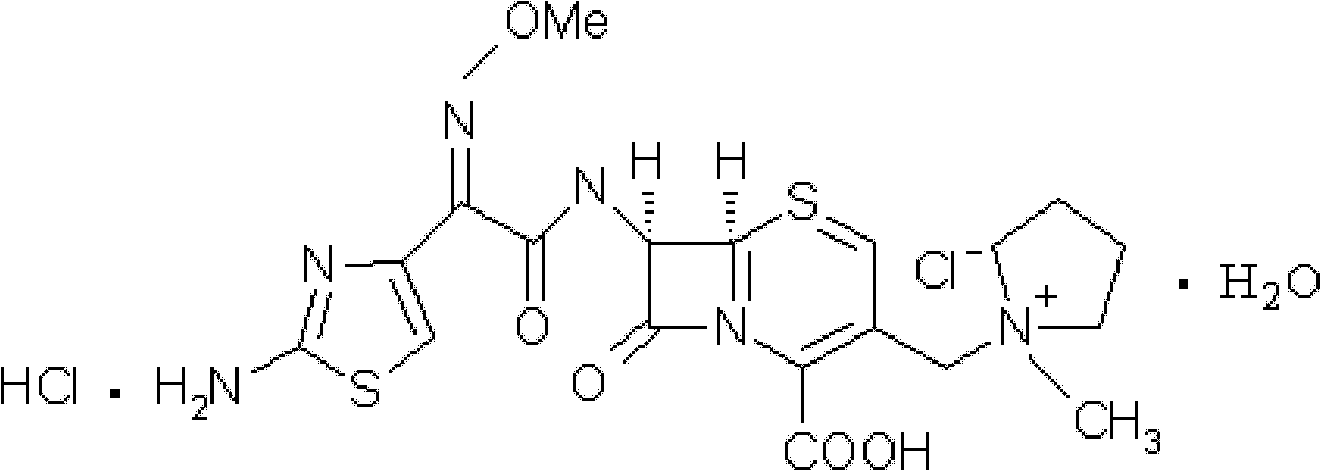
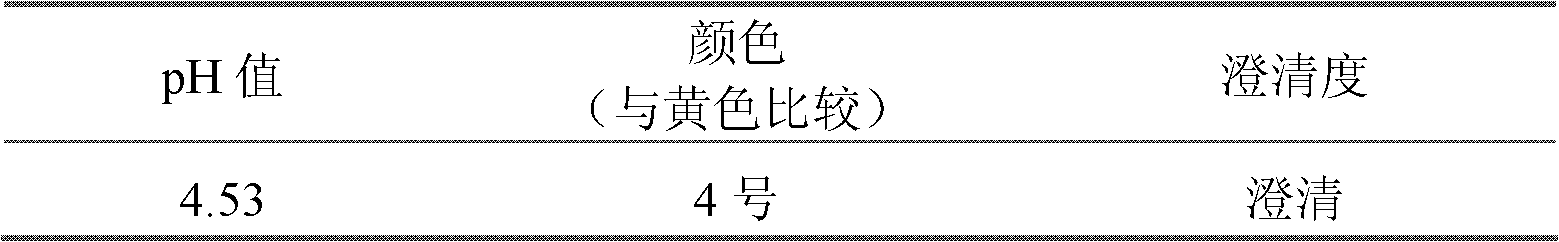
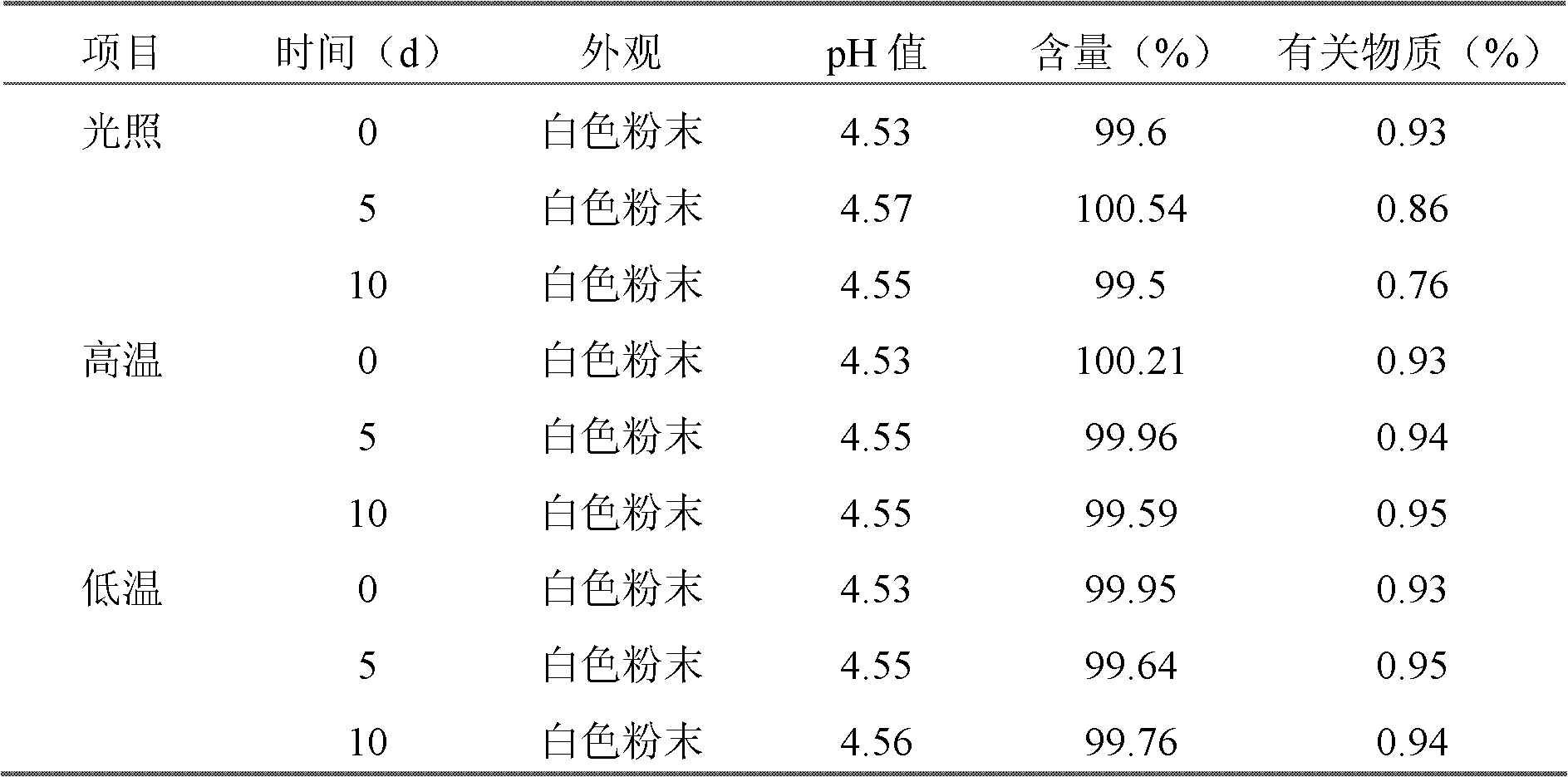
Cefepime hydrochloride composition sterile powder for injection
A technology of cefepime hydrochloride and sterile powder, which is applied in the fields of powder delivery, antibacterial drugs, and medical preparations containing active ingredients, etc. It can solve the problem of low refillable rate and affect the uniformity of cefepime hydrochloride powder injection To improve the uniformity of mixing and improve the uniformity of packaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] [embodiment 1] the preparation of cefepime hydrochloride crystal
[0041] 1) under water bath and stirring conditions, cefepime hydrochloride is dissolved in water to obtain an aqueous solution of cefepime hydrochloride;
[0042] 2) adding activated carbon to the aqueous solution of cefepime hydrochloride obtained in step 1), stirring, suction filtration, and getting the filtrate;
[0043] 3) adjust the stirring speed, and add the mixed solution of precooled ethanol and ether to the filtrate;
[0044] 4) After adding the mixed solution of ethanol and ether, add an ice-water bath to cool down, and stir to grow crystals;
[0045] 5) suction-filtering the crystallization feed liquid, washing, and vacuum-drying to obtain cefepime hydrochloride crystals.
[0046] The bulk density of gained cefepime hydrochloride crystal is 0.3g / cm 3 .
Embodiment 2
[0047] [embodiment 2] the preparation of cefepime hydrochloride crystal
[0048] 1) Dissolve 50 g of cefepime hydrochloride in 100 ml of water at a water bath temperature of 50° C. under stirring conditions to obtain an aqueous solution of cefepime hydrochloride;
[0049] 2) Add 0.5 g of activated carbon to the aqueous solution of cefepime hydrochloride obtained in step 1), stir, suction filter, and get the filtrate;
[0050] 3) Adjust the stirring speed to 250r / min, and add a mixed solution of ethanol and ether that has been pre-cooled to 5°C and has a volume ratio of 1:1 to the filtrate;
[0051] 4) After adding the mixed solution of ethanol and ether, add an ice-water bath to cool down to 0°C, and stir to grow the crystal;
[0052] 5) suction-filtering the crystallization feed liquid, washing, and vacuum-drying to obtain cefepime hydrochloride crystals.
[0053] The bulk density of gained cefepime hydrochloride crystal is 0.5g / cm 3 .
Embodiment 3
[0054] [embodiment 3] the preparation of cefepime hydrochloride crystal
[0055] 1) Dissolve 50 g of cefepime hydrochloride in 75 ml of water at a water bath temperature of 60° C. under stirring conditions to obtain an aqueous solution of cefepime hydrochloride;
[0056] 2) Add 0.45 g of activated carbon to the aqueous solution of cefepime hydrochloride obtained in step 1), stir, suction filter, and get the filtrate;
[0057] 3) Adjust the stirring speed to 400r / min, and add a mixed solution of ethanol and ether that has been pre-cooled to 10°C in a volume ratio of 1:2 to the filtrate;
[0058] 4) After adding the mixed solution of ethanol and ether, add an ice-water bath to cool down to 5°C, and stir to grow crystals;
[0059] 5) suction-filtering the crystallization feed liquid, washing, and vacuum-drying to obtain cefepime hydrochloride crystals.
[0060] The bulk density of gained cefepime hydrochloride crystal is 0.4g / cm 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com