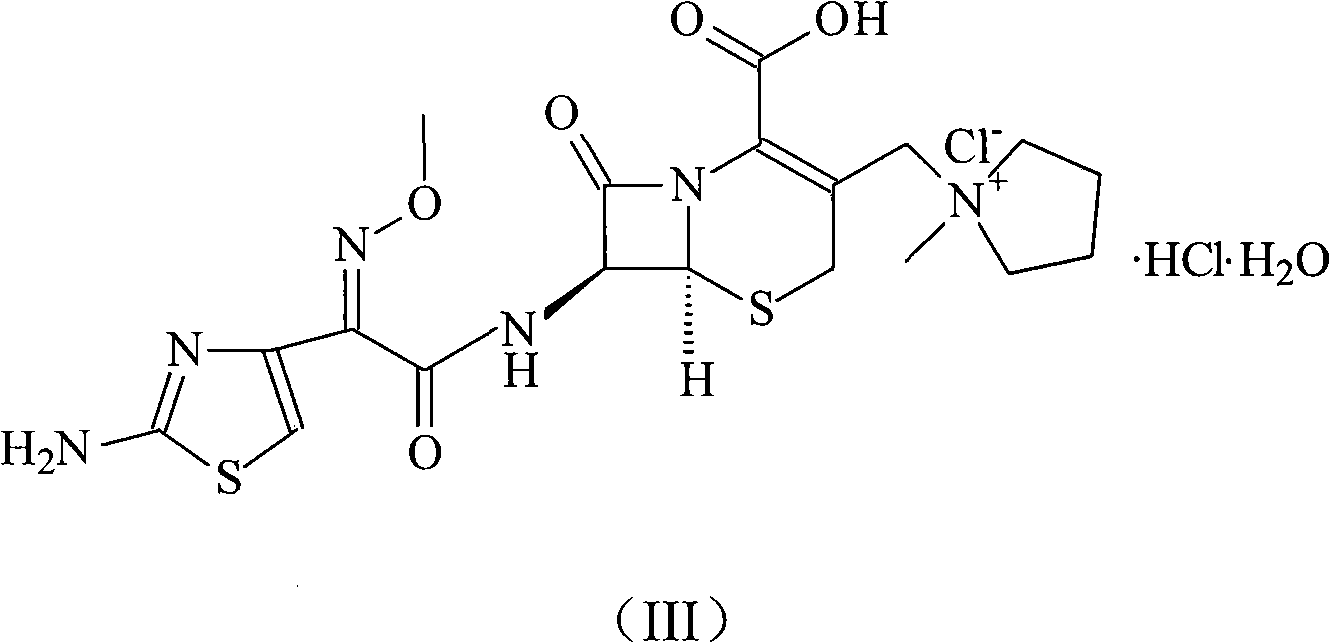
Cefepime hydrochloride compound prepared by new synthetic method
A technology of cefepime hydrochloride and a synthesis method, which is applied in the directions of organic compound/hydride/coordination complex catalyst, chemical instrument and method, chemical/physical process, etc. , low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
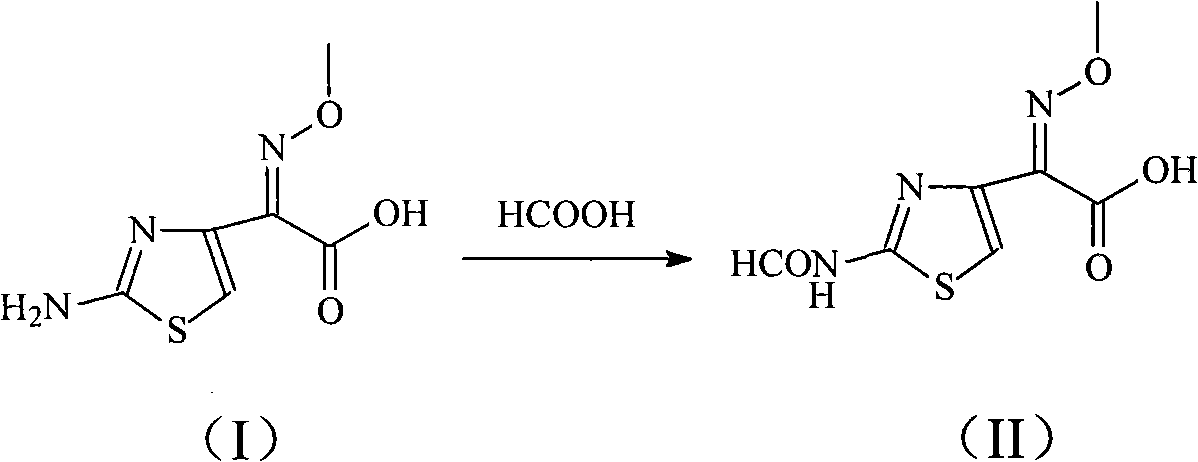
[0035] The synthesis of embodiment 12-(2-formylaminothiazol-4-yl)-2-methoxyimine acetic acid
[0036] Add 201 g of 2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid into 2L of formic acid, and at the same time add 30 g of 4A molecular sieve as a catalyst, heat up to 60 ° C for 4 hours, and distill under reduced pressure to remove most of the Formic acid, add 1L of water, stir, extract with 500ml of ethyl acetate, dry over anhydrous sodium sulfate, concentrate under reduced pressure to get 2-(2-formylaminothiazol-4-yl)-2-methoxyiminoacetic acid 217g, yield 95%.
Embodiment 2
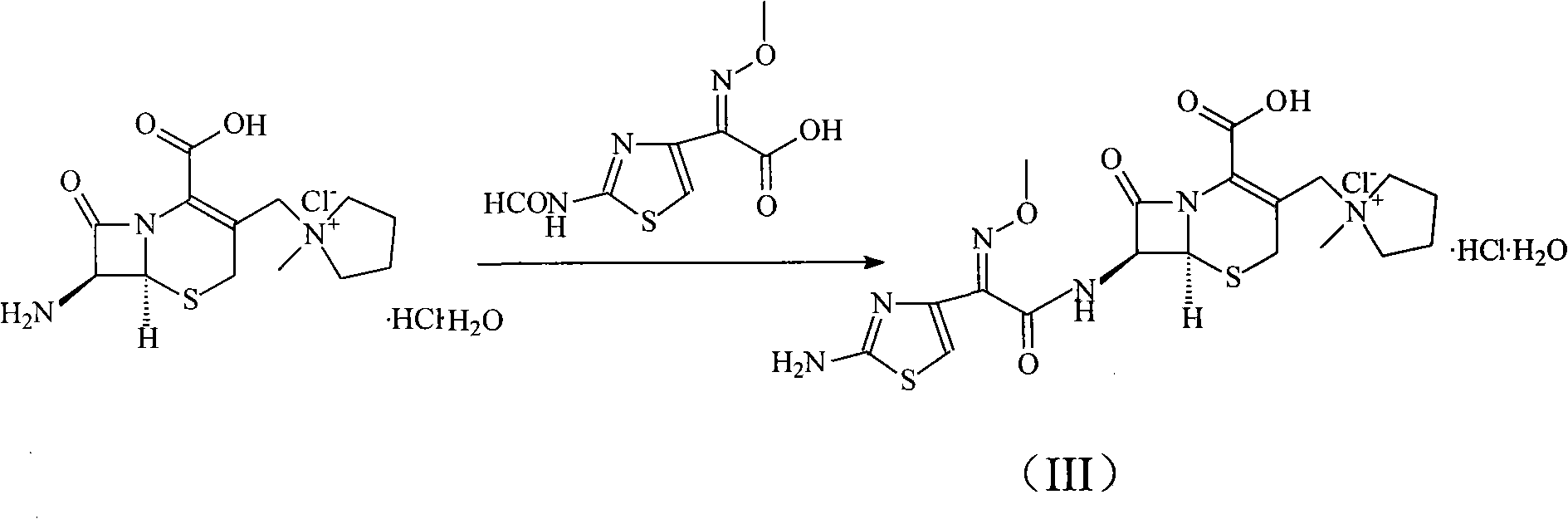
[0037] The synthesis of embodiment 2 cefepime hydrochloride
[0038] 100g of 2-(2-formylaminothiazol-4-yl)-2-methoxyimine acetic acid and 80ml of N,N-diisopropylethylamine were added to 400ml of N,N-dimethylformaldehyde In the amide, cool the reactant to 10°C, add 86g (0.45mol) p-toluenesulfonyl chloride, keep this temperature and stir for 1 hour, then add 187g (0.44mol) 7-MPYCA and 260ml triethylamine, in 5-10 Stir vigorously at ℃ for 30 minutes, then add 4L of acetone and stir to precipitate a solid, filter, wash with 1L of acetone or dichloromethane, and dry under vacuum at 40°C to obtain 223.6 g of cefepime hydrochloride, with a yield of 94%.
[0039] Product Structural Characterization
[0040] 1. Melting point: 150°C.
[0041] 2. Elemental analysis:
[0042] Theoretical value C: 39.9%, H: 4.9%, O: 16.8%, Cl: 12.4%, N: 14.7%, S: 11.2%
[0043] Found C: 39.3%, H: 4.7%, O: 17.2%, Cl: 12.6%, N: 14.4%, S: 11.6%
[0044] It can be confirmed by the above elemental analysis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com