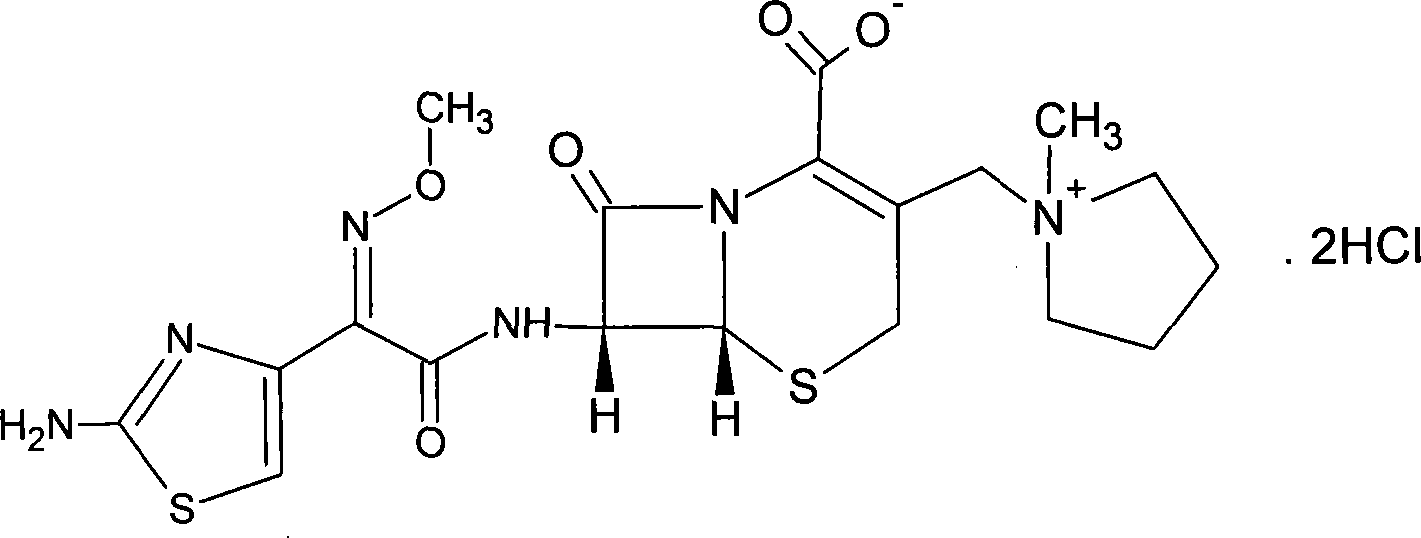
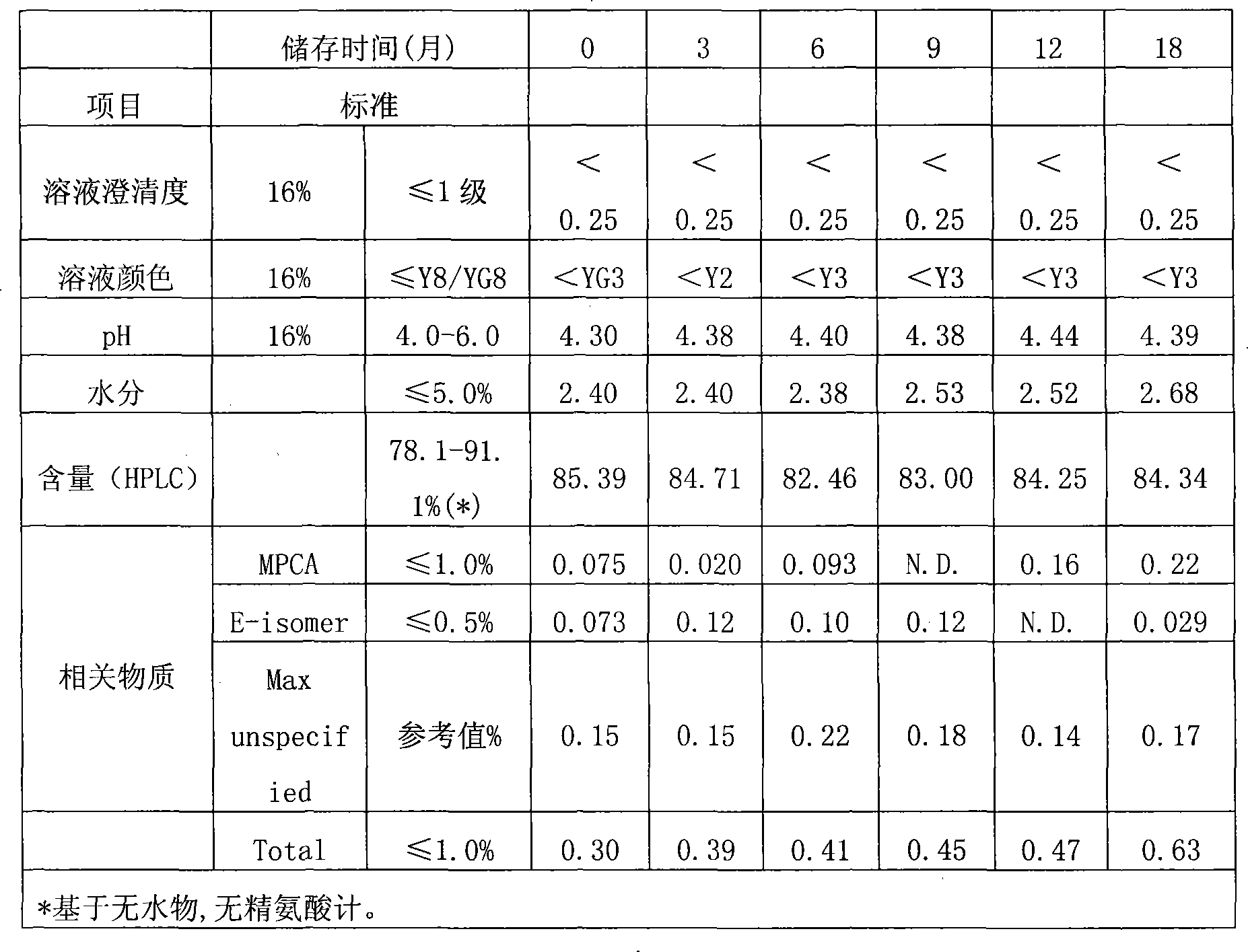
Method for preparing cefepime dihydrochloride and L-arginine mixed powder
A technology of cefepime hydrochloride and arginine, which is applied in the field of medicine and can solve the problems of density, powder fluidity, powder mixing uniformity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0011] A preparation method of cefepime hydrochloride and L-arginine mixed powder is characterized in that cefepime hydrochloride and L-arginine are dissolved to obtain a uniform product through freeze-drying process, and the specific steps are as follows:
[0012] (1) temperature control 0~20 ℃, cefepime hydrochloride and L-arginine by weight ratio (1.65-1.70): 1 make the aqueous solution of 20-30%wt, adjust feed liquid pH to 4.0-6.0, Filter with a 0.22 μm filter membrane into a freeze-drying box;
[0013] (2) Cool down to -45~-55℃ or lower within 2-3 hours, and keep it for 2-3 hours;
[0014] (3) Turn on the vacuum and reach a pressure of 0.10mbar to 0.30mbar for vacuum drying. Raise the set temperature to -10~0°C and keep it for at least 2 hours, then raise the set temperature to 10~20°C and keep it for at least 10 hours To ensure complete sublimation of water;
[0015] (4) Heating up to 30-45°C and drying until the moisture content is less than 3.0%wt.
[0016] The cefe...
Embodiment 1
[0021] Control the temperature at 0-5°C, add 35g of cefepime hydrochloride to 200ml of water for injection, dissolve and add 20.72g of L-arginine to adjust the pH of the feed solution to 4.42, and filter it through a 0.22μm filter membrane into a freeze-drying box. Cool down to -55°C in 2-3 hours and keep for 2 hours. Turn on the vacuum and reach 0.10-0.30mbar for vacuum drying, raise the set temperature to -10°C and keep it for 3 hours, then raise the set temperature to 15°C and keep it for 12 hours to completely sublimate the water. The temperature was raised to 45°C and dried for 3 hours, and the moisture was detected to be 2.17%, and the temperature was lowered to 20°C to obtain 54.21 g of the product.
Embodiment 2
[0023] Control the temperature at 10-15°C, add 14g of cefepime hydrochloride monohydrate to 70ml of water for injection, add 8.48g of L-arginine after dissolving to adjust the pH of the feed solution to 5.85, and filter it through a 0.22μm filter membrane into a freeze-drying box . Cool down to -50°C in 2-3 hours and keep for 3 hours. Turn on the vacuum and reach 0.10-0.30mbar for vacuum drying, raise the set temperature to 0°C and keep it for 2 hours, then raise the set temperature to 20°C and keep it for 10 hours to ensure the water sublimation is complete. Raise the temperature to 40°C and dry for 4 hours to detect that the moisture content is 1.96%, then cool down to 20°C to obtain 21.69 g of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com