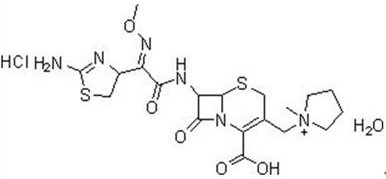
Synthesis method of cefepime hydrochloride
A technology of cefepime hydrochloride and a synthesis method, which is applied in the synthesis field of cefepime hydrochloride, can solve problems such as troublesome processing and reduced yield, and achieve the effects of low reagent cost, simple processing and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Control the temperature at 25°C, add 8.95g of triethylamine and 20g of 7-amino-3-chloromethyl-3-cephalosporin-4-carboxylic acid benzhydryl hydrochloride into 100ml of tetrahydrofuran, stir to dissolve, drop into 9.66 g of di-tert-butyl dicarbonate was stirred under temperature control for 3 hours, then extracted with water and dichloromethane to obtain a dichloromethane solution of the Boc protected product.
[0025] 2) Add 3.77 g of N-methylpyrrolidine to the dichloromethane solution of the Boc-protected product at 25°C, and stir under temperature control for 3 hours. After the reaction is completed, the solvent is spin-dried, and the crude product is stirred with diethyl ether, crystallized and filtered to obtain the inner salt compound.
[0026] 3) At 0°C, the internal salt product obtained in step 2 was stirred in 50ml methanol and 3M HCl to remove the carboxyl and amino protecting groups. After the reaction was completed, continue to add 50ml methanol, stir and f...
Embodiment 2
[0029] 1) Control the temperature at 25°C, add 8.95g of triethylamine and 20g of 7-amino-3-chloromethyl-3-cephalosporin-4-carboxylic acid benzhydryl hydrochloride into 100ml of tetrahydrofuran, stir to dissolve, drop into 9.66 g of di-tert-butyl dicarbonate was stirred under temperature control for 3 hours, then extracted with water and dichloromethane to obtain a dichloromethane solution of the Boc protected product.
[0030] 2) Add 3.77 g of N-methylpyrrolidine to the dichloromethane solution of the Boc-protected product at 25°C, and stir under temperature control for 3 hours. After the reaction is completed, the solvent is spin-dried, and the crude product is stirred with diethyl ether, crystallized and filtered to obtain the inner salt compound.
[0031] 3) At -10°C, stir the internal salt product obtained in step 2 in 50ml methanol and 3M HCl to remove the carboxyl and amino protecting groups. After the reaction is completed, continue to add 50ml methanol, stir and filter ...
Embodiment 3
[0034] 1) Control the temperature at 25°C, add 8.95g of triethylamine and 20g of 7-amino-3-chloromethyl-3-cephalosporin-4-carboxylic acid benzhydryl hydrochloride into 100ml of tetrahydrofuran, stir to dissolve, drop into 9.66 g of di-tert-butyl dicarbonate was stirred under temperature control for 3 hours, then extracted with water and dichloromethane to obtain a dichloromethane solution of the Boc protected product.
[0035] 2) Add 3.77 g of N-methylpyrrolidine to the dichloromethane solution of the Boc-protected product at 25°C, and stir under temperature control for 3 hours. After the reaction is completed, the solvent is spin-dried, and the crude product is stirred with diethyl ether, crystallized and filtered to obtain the inner salt compound.
[0036] 3) At 10°C, stir the internal salt product obtained in step 2 in 50ml methanol and 3M HCl to remove the carboxyl and amino protecting groups. After the reaction is completed, continue to add 50ml methanol, stir and filter t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com