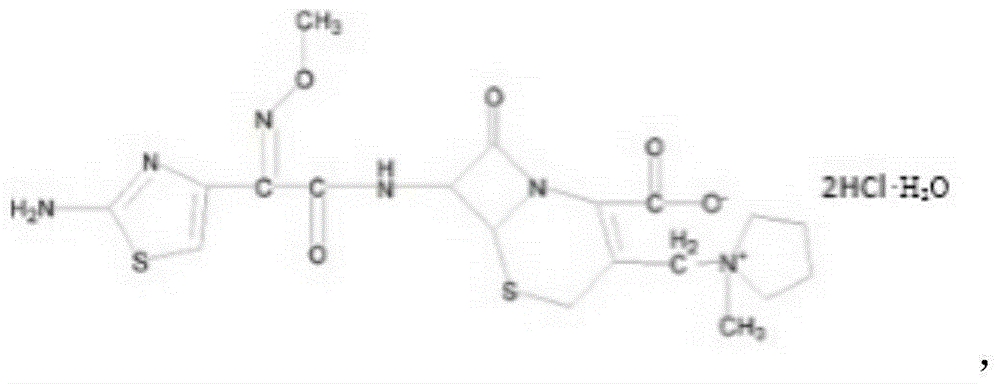
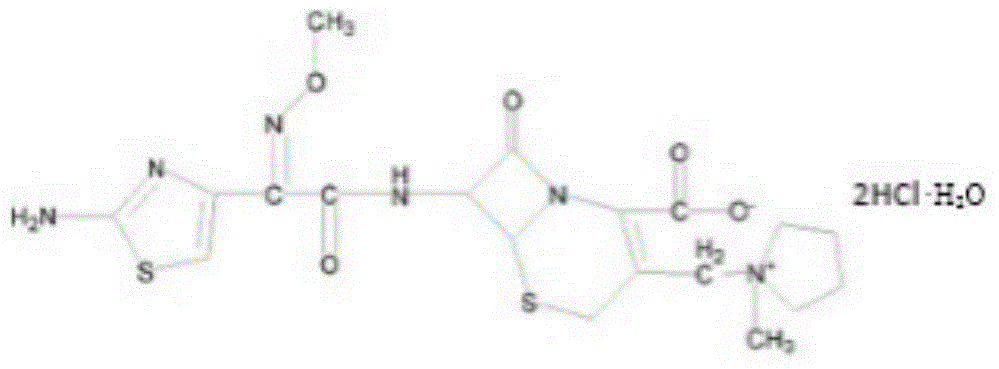
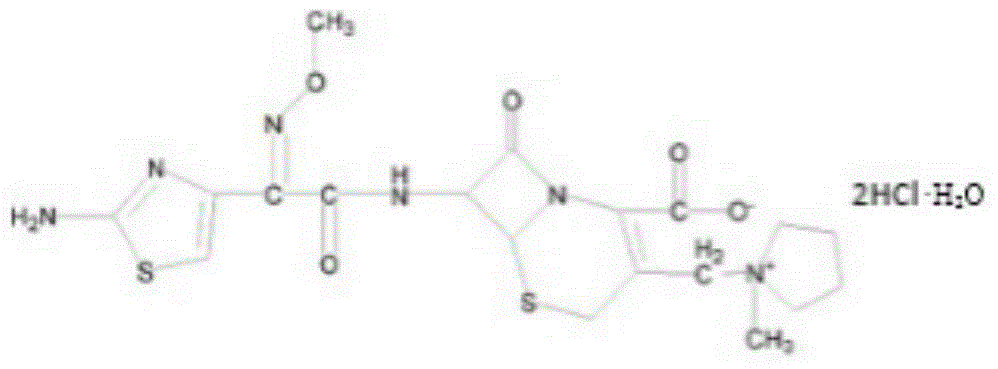
Cefepime dihydrochloride compound and pharmaceutical composition thereof
A technology of cefepime hydrochloride and compounds, which is applied in antibacterial drugs, organic chemistry, and pharmaceutical formulations, can solve the problems of low product purity, increased impurities, and decreased content, and achieve high yield, simple process, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A cefepime hydrochloride compound prepared by the following method:
[0027] (1), 100g of cefepime hydrochloride crude product (purity 83.2%) was dissolved in 1500ml of water, and a separation membrane with a molecular weight cut-off of 500-3000 was selected, and the aromatic polyamide membrane was separated and removed under the operating pressure condition of 0.3MPa;
[0028] (2), the cefepime hydrochloride solution of step (1) was added to 1000ml of a mixed solvent of cyclohexane and acetonitrile with a volume ratio of 1:2, 7.5g of activated carbon was added to the resulting solution, and the temperature was kept at 45°C and stirred for 30min , filter and decarbonize, and collect the filtrate;
[0029] (3), the filtrate of step (2) is carried out chromatographic column separation and purification; The condition of described chromatographic column separation and purification is: mobile phase is the mixed solvent of isopropanol and acetonitrile of volume ratio 1: 3, an...
Embodiment 2
[0040] A cefepime hydrochloride compound prepared by the following method:
[0041] (1), 100g of cefepime hydrochloride crude product (purity 82.4%) was dissolved in 1500ml of water, and a separation membrane with a molecular weight cut-off of 500-3000 was selected, and the sulfonated polysulfone membrane was separated and removed under the operating pressure condition of 0.1MPa;
[0042] (2), the cefepime hydrochloride solution of step (1) was added to 1000ml of a mixed solvent of cyclohexane and acetonitrile with a volume ratio of 1:2, 2.5g of activated carbon was added to the resulting solution, and the temperature was kept at 40°C and stirred for 20min , filter and decarbonize, and collect the filtrate;
[0043] (3), the filtrate of step (2) is carried out chromatographic column separation and purification; The condition of described chromatographic column separation and purification is: mobile phase is the mixed solvent of isopropanol and acetonitrile of volume ratio 2:5,...
Embodiment 3
[0054] A cefepime hydrochloride compound prepared by the following method:
[0055] (1), 100g cefepime hydrochloride crude product (purity 81.7%) is dissolved in 1500ml water, select the separation membrane that molecular weight cut off is 500~3000 for use, under 0.5MPa operating pressure condition, polysulfone membrane separates and removes impurities;
[0056] (2), the cefepime hydrochloride solution of step (1) is added to 1000ml volume ratio is in the mixed solvent of cyclohexane and acetonitrile of 1:2, the gac of 10g is added in the gained solution, is incubated at 50 ℃ and stirred for 25min, Filter and decarbonize, collect the filtrate;
[0057] (3), the filtrate of step (2) is carried out chromatographic column separation and purification; The condition of described chromatographic column separation and purification is: mobile phase is the mixed solvent of isopropanol and acetonitrile of volume ratio 1: 4, and stationary phase filler is aluminum oxide , the flow rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com