Cefepime dihydrochloride preparation method suitable for industrial production
A technology for cefepime hydrochloride and cephalosporin, which is applied in the field of preparation of cefepime hydrochloride, can solve the problems of inability to realize industrialized production, high production equipment requirements, and high solvent recovery pressure, and achieves reduction of production cost and environmental protection expenditure, production Low cost and small product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
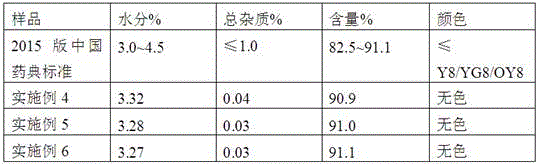
Embodiment 1
[0035] Embodiment 1: the preparation of cefepime hydrochloride crude product
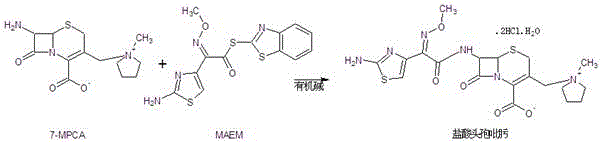
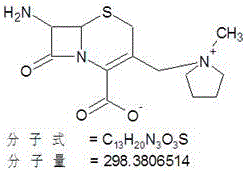
[0036] Put 150L of dichloromethane into the reaction bottle, cool down to 0-5°C, add 20kg (67mol) of 7-MPCA, 25kg (71mol) of MAEM, add 1.5L of 6% sulfurous acid solution, keep the temperature not higher than 5°C, slowly Add 11.2L (80mol) of triethylamine, keep warm at 0-5°C after addition, and react until the residue of 7-MPCA is less than 0.5% (HPLC);
[0037] Put 90L of purified water into the reaction bottle, and separate layers; then use 15L of water to back-extract dichloromethane, combine the water layers, add 1kg of activated carbon, decolorize for 1 hour, filter, wash the filter cake with 10L of water, combine the filtrates, and keep the temperature at 15-25 ℃, add 300L of acetone, 0.05kg of seed crystals, grow the crystals for 2 hours until a large amount of precipitation occurs, then use it for 90-120min, add 390L of acetone, cool down to 0-5℃ and stir for 1 hour. Filtration, washing with...
Embodiment 2
[0038] Embodiment 2: Preparation of crude product of cefepime hydrochloride
[0039] Put 150L of dichloromethane into the reaction bottle, cool down to 0-5°C, add 20kg (67mol) of 7-MPCA, 27kg (77mol) of MAEM, add 0.75L of 6% sulfurous acid solution, keep the temperature not higher than 5°C, slowly Add 10.3L (74mol) of triethylamine, keep warm at 0-5°C after the addition, and react until the residual 7-MPCA is less than 0.5% (HPLC);
[0040] Put 90L of purified water into the reaction bottle, and separate layers; then use 15L of water to back-extract dichloromethane, combine the water layers, add 1kg of activated carbon, decolorize for 1 hour, filter, wash the filter cake with 10L of water, combine the filtrates, and keep the temperature at 15-25 ℃, add 320L of isopropanol, 0.05kg of seed crystals, grow the crystals for 2 hours until a large amount of precipitation occurs, then use it for 90-120min, add 400L of isopropanol, cool down to 0-5℃ and stir for 1 hour. Filtration, wa...
Embodiment 3
[0041] Embodiment 3: Preparation of crude product of cefepime hydrochloride
[0042] Put 150L of dichloromethane into the reaction bottle, cool down to 0-5°C, add 20kg (67mol) of 7-MPCA, 26kg (74mol) of MAEM, add 1L of 6% sulfurous acid solution, keep the temperature not higher than 5°C, and add slowly Diisopropylamine 11.3L (80mol), keep warm at 0-5°C after addition, and react until the residual 7-MPCA is less than 0.5% (HPLC);
[0043]Put 90L of purified water into the reaction bottle, and separate layers; then use 15L of water to back-extract dichloromethane, combine the water layers, add 1kg of activated carbon, decolorize for 1 hour, filter, wash the filter cake with 10L of water, combine the filtrates, and keep the temperature at 15-25 ℃, add 360L of ethanol, 0.05kg of seed crystals, grow crystals for 2 hours until a large amount of precipitation occurs, then use for 90-120min, add 420L of ethanol, cool down to 0-5℃ and stir for 1 hour. Filtration, washing with ethanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com