Cefepime hydrochloride medicine composition, powder-injection thereof and preparation method thereof
A technology of cefepime hydrochloride and its composition, which is applied in the field of drug invention, can solve problems such as pH stability and uneven packaging, and achieve the effects of uniform product quality, good packaging uniformity, and good uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
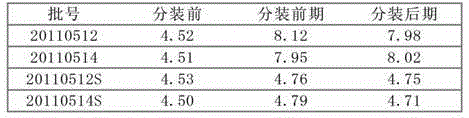
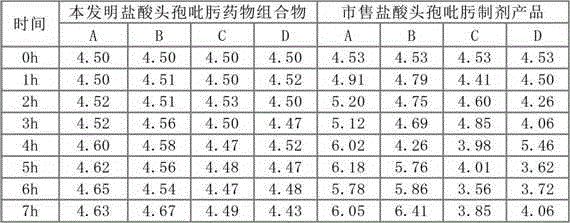
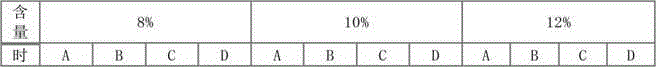
[0035] Embodiment 1: prescription and technology of cefepime hydrochloride pharmaceutical composition
[0036] Cefepime Hydrochloride 100kg
[0037] L-Arginine 40kg
[0038] Hydroxypropyl-β-cyclodextrin 10kg
[0039]Preparation method: respectively crush cefepime hydrochloride, L-arginine and hydroxypropyl-β-cyclodextrin, pass through a 28-mesh sieve, weigh 100 kg of cefepime hydrochloride and 40 kg of L-arginine after sieving 10kg of hydroxypropyl-beta-cyclodextrin, mixed evenly to obtain the cefepime hydrochloride pharmaceutical composition.
Embodiment 2
[0040] Embodiment 2: prescription and technology of cefepime hydrochloride pharmaceutical composition
[0041] Cefepime Hydrochloride 100kg
[0042] L-Arginine 40kg
[0043] Hydroxypropyl-β-cyclodextrin 8kg
[0044] Preparation method: 1) remove the outer package from the raw and auxiliary materials, crush cefepime hydrochloride and L-arginine respectively, and pass through a 28-mesh sieve; 2) take 100 kg of cefepime hydrochloride and 40 kg of L-arginine after sieving, respectively, Mix evenly and add to the coating machine; 3) Dissolve 8kg of hydroxypropyl-β-cyclodextrin in an appropriate amount of ethanol, and spray the resulting solution evenly into the particles of the mixture of cefepime hydrochloride and L-arginine in a fine mist The surface of the layer is dried in a fluidized bed to obtain the cefepime hydrochloride pharmaceutical composition.
Embodiment 3
[0045] Embodiment 3: the prescription of cefepime hydrochloride pharmaceutical composition
[0046] Cefepime Hydrochloride 100kg
[0047] L-Arginine 40kg
[0048] Hydroxypropyl-β-cyclodextrin 12kg
[0049] The preparation method is as follows: 1) remove the outer package from the raw and auxiliary materials, crush cefepime hydrochloride and L-arginine respectively and pass through a 28-mesh sieve; 2) take 100 kg of cefepime hydrochloride and 40 kg of L-arginine after sieving respectively , mixed evenly and added to the coating machine; 3) Dissolve 12kg of hydroxypropyl-β-cyclodextrin in an appropriate amount of ethanol, and spray the resulting solution evenly into the mixture of cefepime hydrochloride and L-arginine in a fine mist The particle layer surface is dried in a fluidized bed to obtain the cefepime hydrochloride pharmaceutical composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com