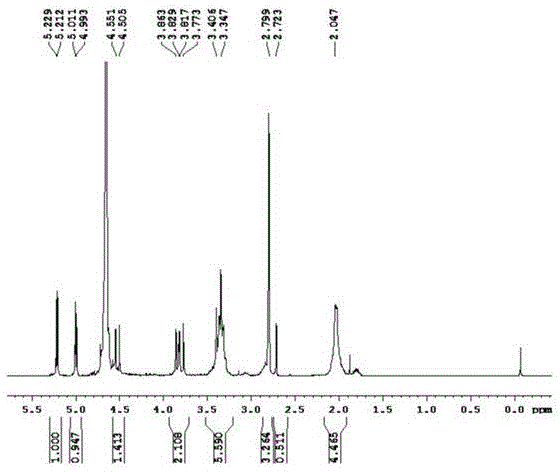
Method for preparing cefepime dihydrochloride
A technology of cefepime hydrochloride and cephalosporin, applied in the field of preparation of cefepime hydrochloride, can solve the problems of insufficient supply, high price, inconvenient post-processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 A preparation method of cefepime hydrochloride. The steps and conditions are as follows:
[0016] The invention provides a preparation method of cefepime hydrochloride. The steps and conditions are as follows:
[0017] (1) The raw materials are: 7-Aminocephalosporanic acid (abbreviated as 7-ACA), hexamethyldisilazane (abbreviated as HMDS), trimethyliodosilane (abbreviated as TMSI), N-methylpyrrolidine (abbreviated as NMP); CH 3 OH (methanol); the mass ratio of raw material components is 7-ACA: HMDS: catalyst: TMSI: NMP: CH 3 OH=1:3:0.02:1.8:3:1; the catalyst is 95% hydrochloric acid;
[0018] (2) The crude product of cefepime hydrochloride was prepared as follows: According to the mass ratio of raw materials, under the protection of dry nitrogen, add 5.0g of 7-ACA to a dry 100mL three-necked flask, press CH 3 OH mass g: the volume of anhydrous dichloromethane mL is 1: 50 Add anhydrous dichloromethane, stir, heat at 45°C for 3 h, add 15 g of HMDS, add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com