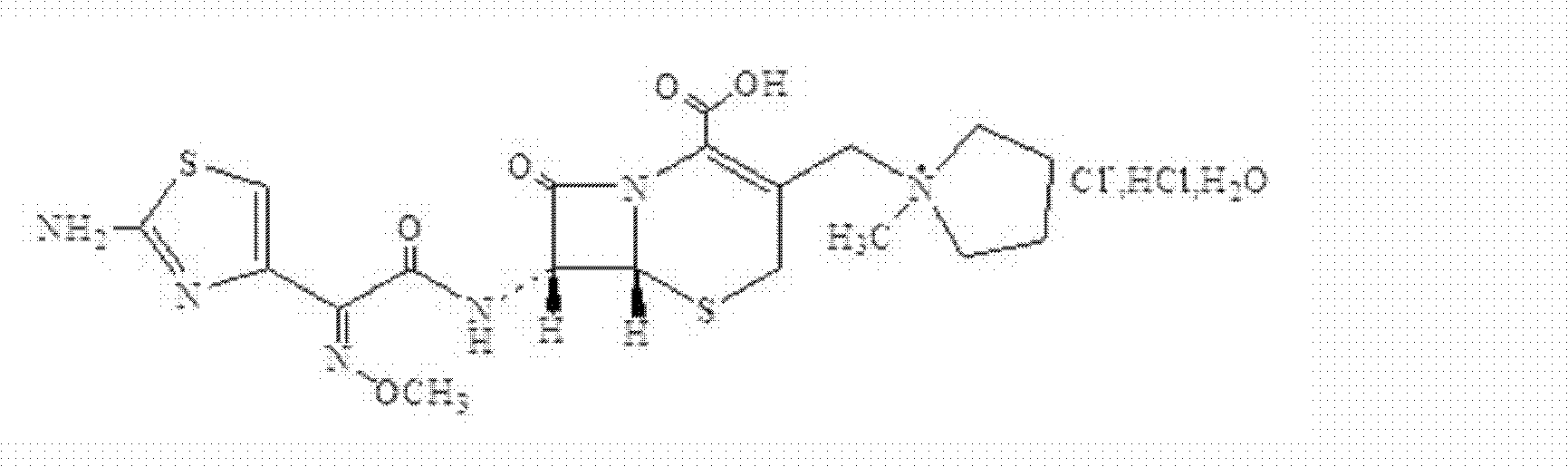
A kind of preparation method of cefepime hydrochloride
A technology of cefepime hydrochloride and aminocephalosporanic acid, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of expensive raw materials, unsuitability for large-scale industrial production, and low yield, and achieve simple operation, improved industrial hygiene environment, and improved conversion rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Dissolve 100g of 7-aminocephalosporanic acid (7-ACA) in 500g of dichloromethane solvent (wet the 7-ACA with dichloromethane before feeding and then use the wet feeding method), add 100g of N, O-bistrimethylsilane Acetamide (BSA), stirred at room temperature for two hours, cooled to 10°C, added 80g N, N-diethylaniline, 120g iodotrimethylsilane, and reacted for 3-4 hours. When 7ACA<1.0g / l is detected by liquid phase, the reaction can be regarded as complete.
[0036] 2. At the end of the reaction, add 50g of N-methylpyrrolidine dropwise. After the dropwise addition, keep the reaction at 0-5°C for 1 hour. If the iodide residue is less than 1.0%, the reaction can be ended.
[0037] 3. Add the above solution to 30g of methanol, stir and react for 1 hour, then add a hydrochloric acid solution of 210g of concentrated hydrochloric acid and 210g of water, the temperature of the feed liquid during the dropwise addition should not exceed 10°C, stir quickly until it is completel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com