Method for synthesizing cefepime hydrochloride
A technology of cefepime hydrochloride and its synthesis method, which is applied in the fields of chemical instruments and methods, organic compound/hydride/coordination complex catalyst, organic chemistry, etc., and can solve unfavorable continuity, large-scale industrial production, and harsh reaction conditions , high cost of raw materials and other issues, to achieve the effect of increasing product yield, improving purity, and improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
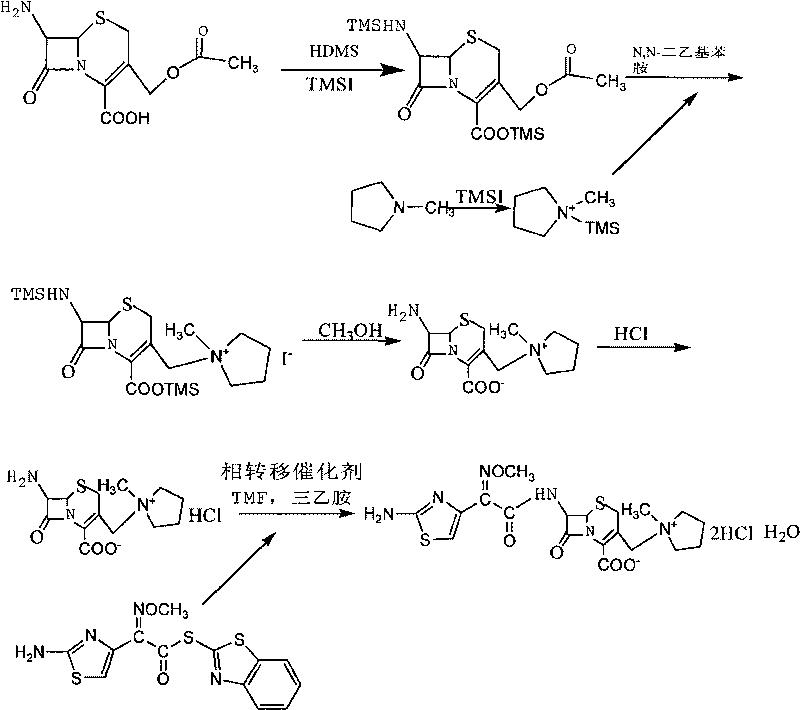
[0022] 1. Preparation of 7-MPCA
[0023] Add 200ml of dichloromethane, 40ml of hexamethyldisilazane, 0.2ml of iodotrimethylsilane, and 40g of 7-ACA into a three-necked flask, stir and mix well, and heat to reflux for 8 hours under nitrogen protection. After detecting that no ammonia gas is released, cool the solution to 0-5°C to obtain solution ①. At the same time, 100ml of dichloromethane, 20.4g of N-methylpyrrolidine, and 26ml of iodotrimethylsilane were added to a dry three-necked flask, and the temperature was kept at 20-25°C for 2 hours. Get the solution ②.
[0024] Mix solution ② and solution ① at 0-5°C, add 11ml of N,N-diethylaniline, react for 2h, after the reaction, add 20ml of methanol, 160ml of hydrochloric acid, 200ml of water, stir for 10 minutes, stand still, and separate The aqueous phase, the organic phase was extracted with water, and the aqueous phases were combined. Add 400ml of acetone to the water phase under stirring, adjust the pH to 2 with triethylam...
Embodiment 2
[0028] 1. Synthesis of 7-MPCA
[0029] In a 500ml round bottom flask, add 40g of 7-ACA, 27.4g of hexamethyldisilazane, and 200ml of cyclohexane in sequence, reflux at 85°C for 6h, then cool for later use. Add 50ml of cyclohexane, 35ml of N-methylpyrrolidine, and 26ml of iodotrimethylsilane into an anhydrous 250ml round-bottomed flask and react at reflux at 70°C for 3h, then cool for later use. Put the above two together into a 1000ml round bottom flask, cool to 0°C and quickly add 10ml of N,N-diethylaniline, after half an hour of reaction at 0°C, add 50ml isopropanol, 350ml water and 150ml concentrated hydrochloric acid dropwise until the solid dissolves. After liquid separation, the organic phase was re-extracted with 200 ml of water, the aqueous phases were combined, acetone was added dropwise to crystallize, and 40.2 g of the product was obtained by suction filtration and drying, with a yield of 82.3%.
[0030] 2, the synthesis of cefepime hydrochloride
[0031] Add 200m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com