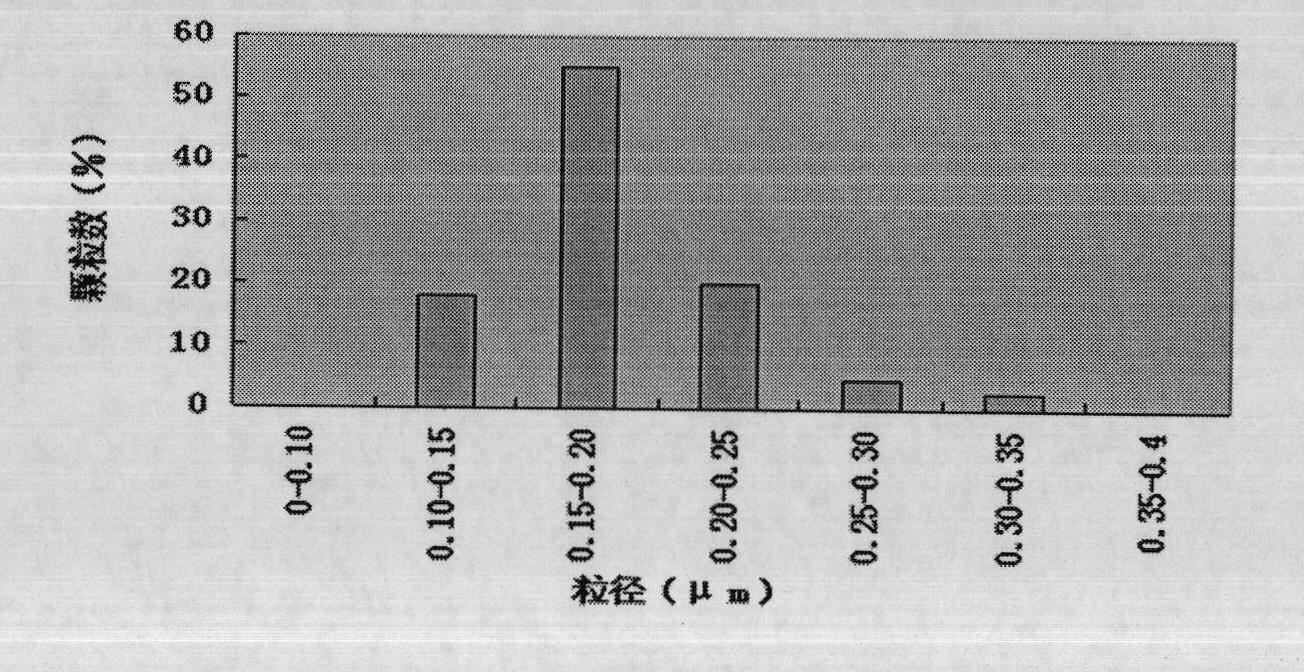
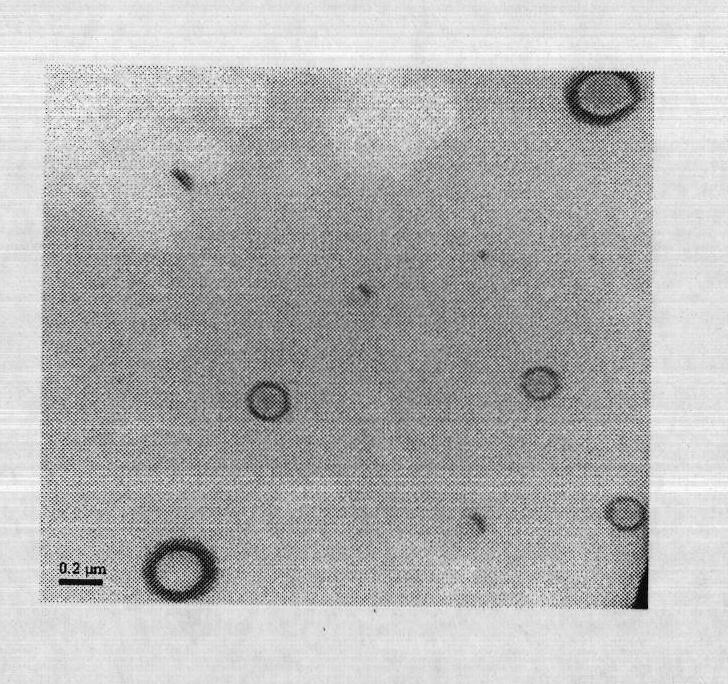
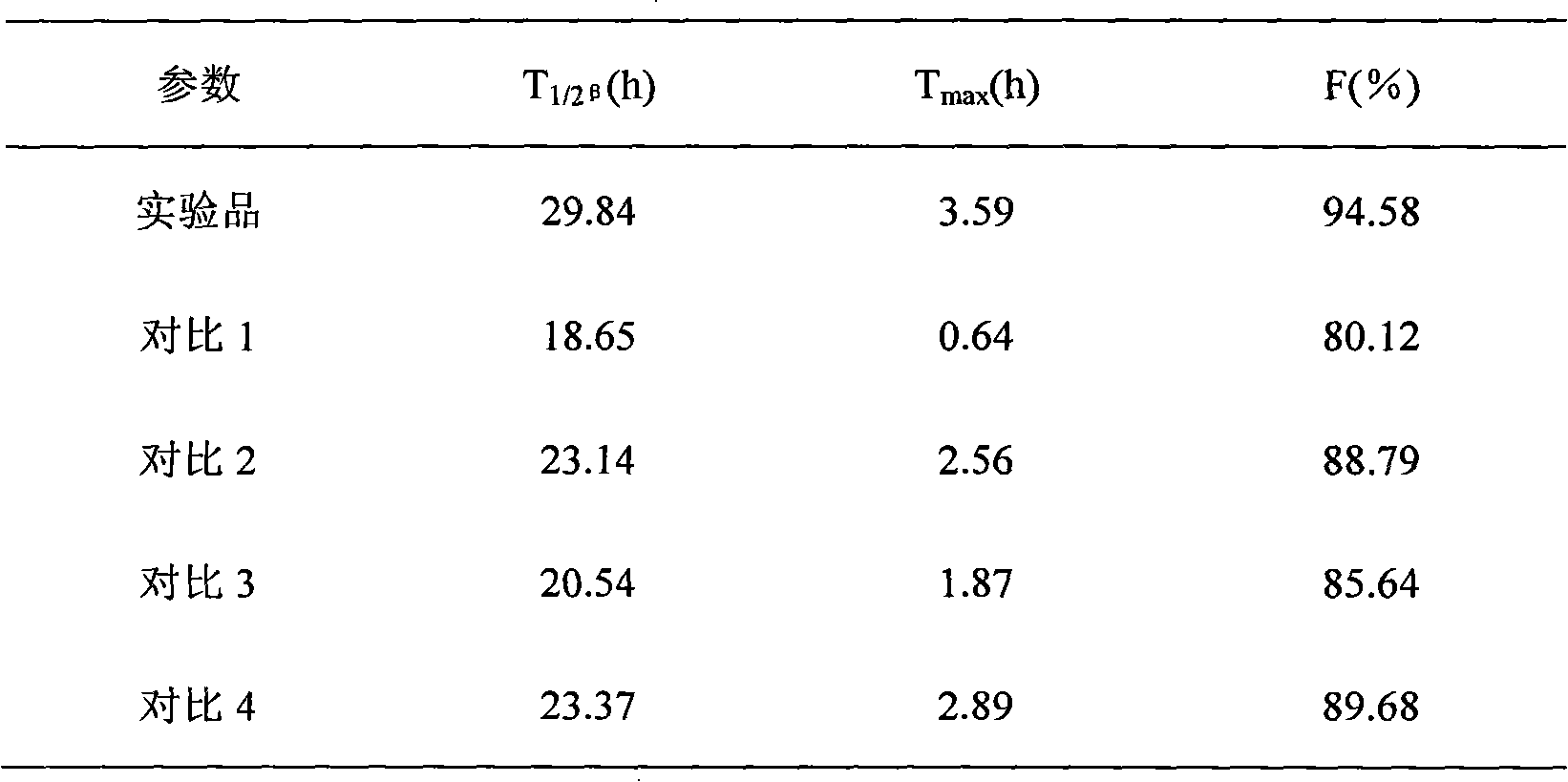
Compound ceftiofur suspension emulsion injection and preparation method thereof
A technology of ceftiofur and ceftiofur hydrochloride, applied in the field of medicine, can solve the problems of serious antibiotic resistance, waste of human and financial resources, inconvenient use, etc., and achieve the effects of improving curative effect, reducing equipment cost and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation technology of embodiment 1 compound recipe ceftiofur suspoemulsion injection is as follows:
[0048] Ceftiofur Hydrochloride 1.5g
[0049] Magnolia Oil 1.5g
[0050] EL-40 10.0g
[0051] Ethyl acetate 5.0g
[0052] Ve oil 0.1g
[0053] Sodium carboxymethylcellulose 0.1g
[0054] Water for injection 81.8g
[0055] The specific operation steps are:
[0056] 1) Filter soybean oil for injection, cool to about 70°C after autoclaving, and weigh 5.0 g for later use;
[0057] 2) Weigh 1.5g of ceftiofur hydrochloride and 1.5g of magnolia oil, slowly add them to the sterilized oil for injection, stir while adding, so that the medicine and oil are fully mixed;
[0058] 3) Weigh 10.0 g of EL-40, stir evenly and add to the oil for injection obtained in step 2).
[0059] 4) Add 0.1 g of Ve oil and 0.1 g of sodium carboxymethylcellulose to the oil for injection obtained in step 3), and stir thoroughly.
[0060] 5) Take 81.8g of water for injection, heat it to ...
Embodiment 2
[0061] The preparation technology of embodiment 2 compound recipe ceftiofur suspoemulsion injection is as follows:
[0062] Ceftiofur Hydrochloride 2.5g
[0063] Magnolia Oil 2.5g
[0064] Tween-80 4.2g
[0065] Span-80 2.8g
[0066] Soybean oil for injection 7.0g
[0067]Ve oil 0.1g
[0068] Sodium carboxymethylcellulose 0.07g
[0069] Water for injection 80.83g
[0070] Concrete operation steps are with embodiment 1.
Embodiment 3
[0071] The preparation process of embodiment 3 compound ceftiofur suspoemulsion injection is as follows:
[0072] Ceftiofur Hydrochloride 5.0g
[0073] Magnolia Oil 5.0g
[0074] Tween-80 3.0g
[0075] Span-80 2.0g
[0076] Soybean oil for injection 10.0g
[0077] Ve oil 0.3g
[0078] Sodium carboxymethylcellulose 0.05g
[0079] Water for injection 74.65g
[0080] Concrete operation steps are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com