Ceftiofur hydrochloride breast injectant during dry period
A technology of ceftiofur hydrochloride and breast injection, which is applied in the formulation and preparation of ceftiofur hydrochloride breast injection in dry lactation period, and can solve the problem of high transportation and storage conditions, lack of protection of dairy cow teats, and easy sedimentation of the preparation and other problems, to achieve good therapeutic and preventive effects, good drug absorption, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
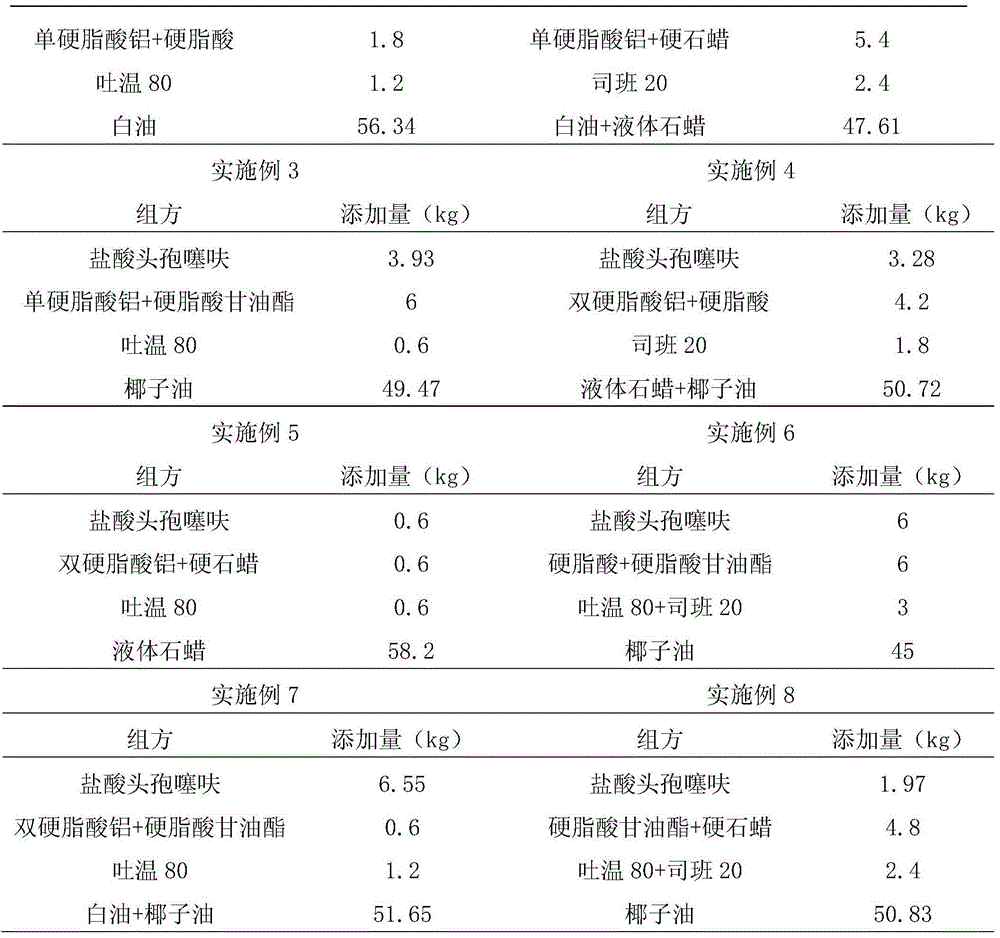
Examples
Embodiment 9
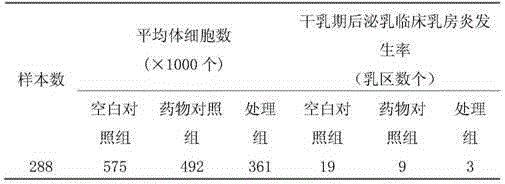
[0032] Embodiment 9: curative effect test
[0033] 1. Materials
[0034] Blank control group: normal saline
[0035] Drug control group: 10% ceftiofur hydrochloride injection, commercially available.
[0036] Test sample group: (Example 7) Ceftiofur hydrochloride breast injection (dry period).
[0037] 2. Method
[0038] Select 24 healthy dairy cows that are about to stop milking, and divide them into 3 groups, 8 in each group; after the last time to squeeze out the residual milk in the udder, the blank control group is injected with normal saline, and the drug control group is injected with 10% ceftiofur hydrochloride. liquid, the test sample group, that is, the treatment group, used the ceftiofur hydrochloride breast injection of the formula in Example 7, and injected the preparation containing 500 mg ceftiofur into the breast pool through the nipple duct.
[0039] 3. Somatic cell detection experiment
[0040] The somatic cell count content in milk can reflect the healt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com