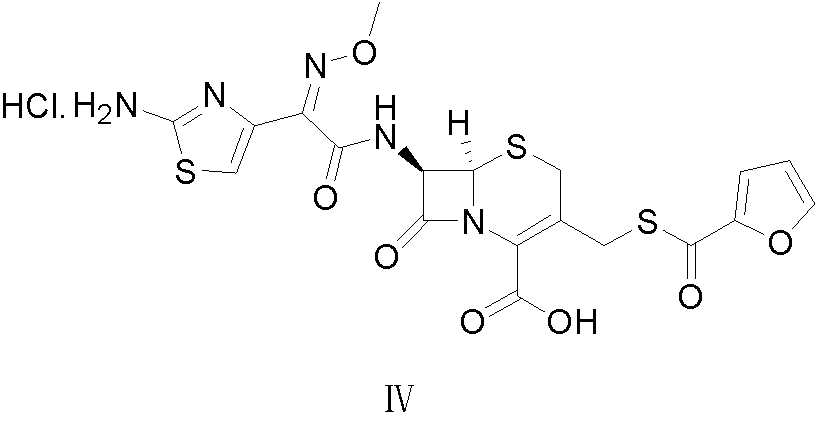
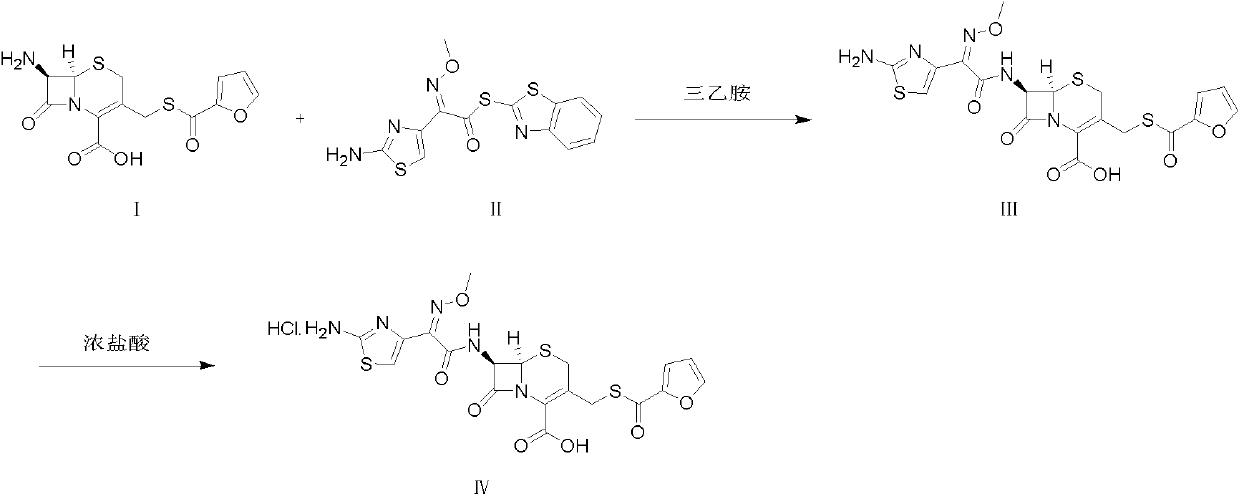
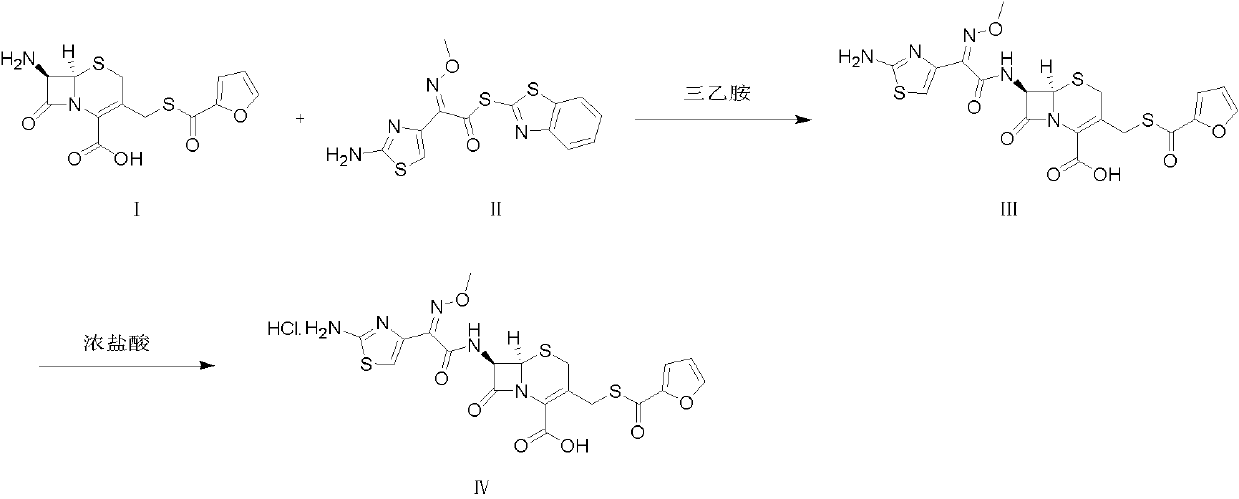
Preparation method of ceftiofur hydrochloride
A technology of ceftiofur hydrochloride and cefuroxime, which is applied in a preparation field of ceftiofur hydrochloride, can solve the problems of environmental pollution, high production cost and high cost, and achieve the effects of reducing environmental pollution and production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthesis of embodiment 1 ceftiofur hydrochloride
[0030] Add 50 g of cefuroxime and 300 mL of dichloromethane into a 1000 mL three-necked reaction flask, cool the mixture to 5°C, add 29.7 g of triethylamine dropwise, and finish adding dropwise in 20 minutes, then add AE-activator in batches after stirring for 20 minutes Add 77 g of water to 77 g of ester, add 15 mL of water, keep stirring at 5°C for 3 hours, extract twice with 300 mL of water, combine the water phases, add 10 g of activated carbon to decolorize for 30 min, filter, add 30 mL of 5 mol / L hydrochloric acid to the filtrate to adjust the pH value to 1.7 , filtered to obtain a solid wet product, which was dissolved in 500 mL of acetone, and 23 mL of 5 mol / L hydrochloric acid was added dropwise under stirring to adjust the pH value to 1.0, and 1750 mL of water was slowly added dropwise, and solids were gradually precipitated, stirred for 30 min, and left to stand After 2 h, it was filtered, and the solid ...
Embodiment 2
[0031] The synthesis of embodiment 2 ceftiofur hydrochloride
[0032] Add 40 g of cefuroxime and 240 mL of dichloromethane into a 1000 mL three-necked reaction flask, cool the mixture to 0°C, add 17.8 g of triethylamine dropwise, finish adding dropwise in 20 minutes, and add AE-activator in batches after stirring for 20 minutes Add 12 mL of water to 41.2 g of ester, keep stirring at 0°C for 3 hours, extract twice with 250 mL of water, combine the water phases, add 8 g of activated carbon to decolorize for 30 min, filter, add 23 mL of 5 mol / L hydrochloric acid to the filtrate to adjust the pH value 1.7, filter to obtain a solid wet product, dissolve it in 640 mL of acetone, add 18 mL of 5 mol / L hydrochloric acid dropwise under stirring to adjust the pH value to 1.0, slowly add 1790 mL of water dropwise, gradually a solid precipitates out, stir for 30 min, Let stand for 2 h, filter, and dry the solid at 40°C to obtain 34.6 g of white solid powder of ceftiofur hydrochloride, with...
Embodiment 3
[0033] The synthesis of embodiment 3 ceftiofur hydrochloride
[0034] Add 40 g of cefuroxime and 240 mL of dichloromethane into a 1000 mL three-necked reaction flask, cool the mixture to 10°C, add 25.4 g of diethylamine dropwise, finish adding dropwise in 20 minutes, and add AE-Active in batches after stirring for 20 minutes Add 12 mL of water to 61.7 g of ester, keep stirring at 10°C for 3 hours, extract twice with 250 mL of water, combine the water phases, add 8 g of activated carbon to decolorize for 30 min, filter, add 28 mL of 5 mol / L hydrochloric acid to the filtrate to adjust the pH value 1.7, filter to obtain a solid wet product, dissolve it in 320 mL of acetone, add 18 mL of 5 mol / L hydrochloric acid dropwise under stirring to adjust the pH value to 1.4, slowly add 2560 mL of water dropwise, gradually a solid precipitates out, stir for 30 min, Let stand for 2 h, filter, and dry the solid at 40°C to obtain 48.3 g of white solid powder of ceftiofur hydrochloride, with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com