Ceftiofur hydrochloride lipidosome injection and preparation method thereof
The technology of ceftiofur hydrochloride and cephalosporin hydrochloride is applied in the field of ceftiofur hydrochloride liposome preparation and preparation of ceftiofur hydrochloride liposome injection, which can solve the problems of poor compliance and frequent administration, and achieve the encapsulation efficiency High, slow release effect, uniform particle size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
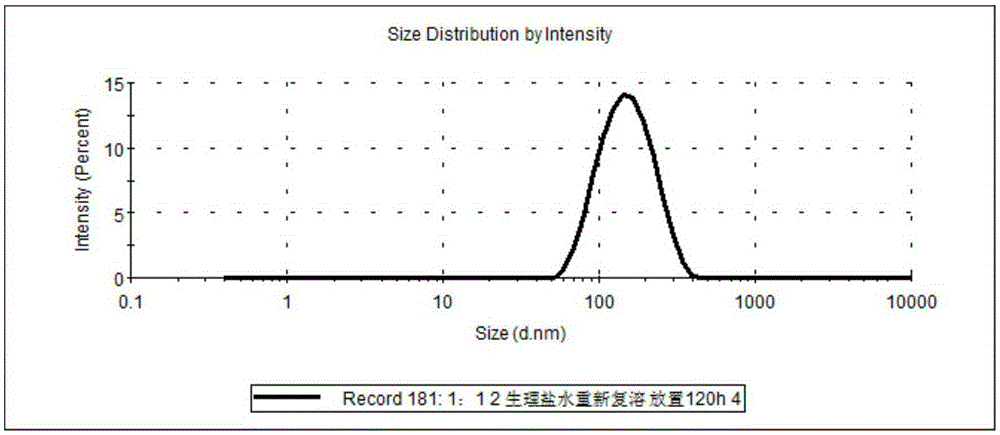
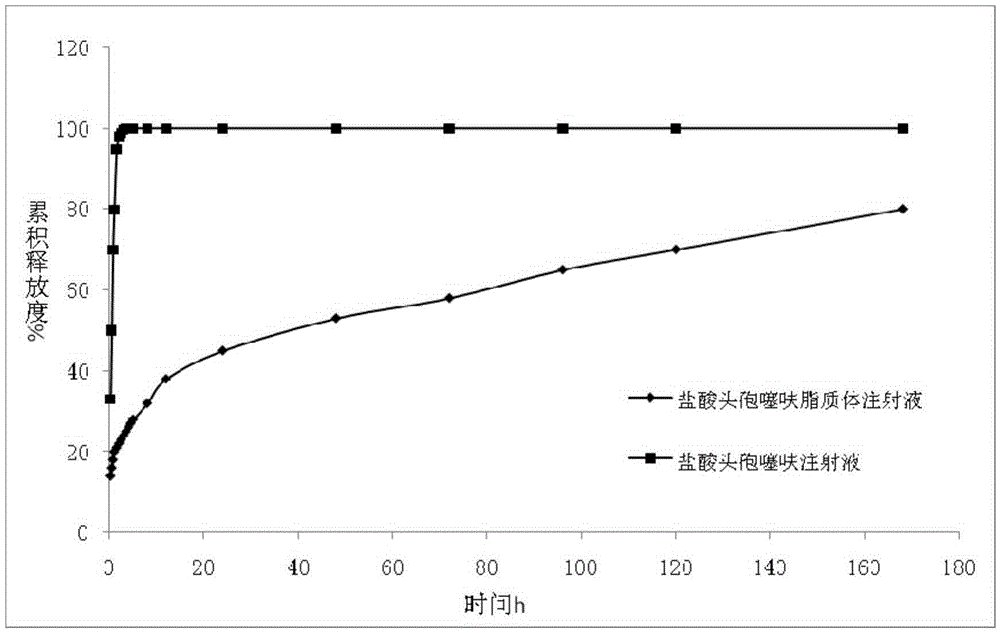
Image
Examples
Embodiment 1
[0031] Prescription (Specification 10%)
[0032] Ceftiofur Hydrochloride 100g
[0033] Lecithin Niacinamide 100g
[0034] Cholesterol 100g
[0035] Bianze-78 10g
[0036] Sodium dihydrogen phosphate 30g
[0037] Disodium hydrogen phosphate 250g
[0038] Vitamin E 30g
[0039] Dichloromethane 200ml
[0040] Water for injection 1L
[0041] Preparation Process:
[0042] 1) Dissolve 30 g of sodium dihydrogen phosphate and 250 g of disodium hydrogen phosphate in 1 L of water for injection to prepare a phosphate buffer, and adjust the pH to 6 with 0.1 mol / L of sodium hydroxide solution and 2% phosphoric acid. 100g of ceftiofur hydrochloride was dissolved in phosphate buffered saline to obtain an aqueous phase;
[0043]2) Dissolve 100g lecithin nicotinamide, 100g cholesterol and 10g Bianze-78 in dichloromethane to obtain an organic phase. Add the organic phase to the water phase under the condition of heating in a water bath at 50-60°C, mix evenly, and shear at high speed w...
Embodiment 2
[0046] Prescription (Specification 10%)
[0047] Ceftiofur Hydrochloride 100g
[0048] Hydrogenated Soy Phosphatidylcholine (HSPC) 909g
[0049] Cholesterol 91g
[0050] Cholesterol-PEG2000 50g
[0051] Potassium dihydrogen phosphate 150g
[0052] Dipotassium hydrogen phosphate 10g
[0054] Chloroform 500ml
[0055] Water for injection 1L
[0056] Preparation Process:
[0057] 1) Dissolve 150 g of potassium dihydrogen phosphate and 10 g of dipotassium hydrogen phosphate in 1 L of water for injection to prepare a phosphate buffer solution, and adjust the pH to 7.5 with 0.1 mol / L sodium hydroxide solution and 2% phosphoric acid. 100g of ceftiofur hydrochloride was dissolved in phosphate buffered saline to obtain an aqueous phase;
[0058] 2) 909g of hydrogenated soybean phosphatidylcholine (HSPC), 91g of cholesterol and 50g of cholesterol-PEG2000 were dissolved in chloroform to obtain an organic phase. Add the organic phase to the water pha...
Embodiment 3
[0061] Prescription (Specification 10%)
[0062] Ceftiofur Hydrochloride 100g
[0063] Distearoylphosphorylcholine (DSPC) 416.7g
[0064] Cholesterol 83.3g
[0065] Cholesterol-PEG1000 30g
[0066] Potassium dihydrogen phosphate 80g
[0067] Dipotassium hydrogen phosphate 200g
[0068] Sodium metasulfite 80g
[0069] Absolute ethanol 100ml
[0070] Water for injection 1L
[0071] Preparation Process:
[0072] 1) Dissolve 80 g of potassium dihydrogen phosphate and 200 g of dipotassium hydrogen phosphate in 1 L of water for injection to prepare a phosphate buffer, and adjust the pH to 6 with 0.1 mol / L of sodium hydroxide solution and 2% phosphoric acid. 100g of ceftiofur hydrochloride was dissolved in phosphate buffered saline to obtain an aqueous phase;
[0073] 2) Dissolve 416.7g of distearoylphosphorylcholine (DSPC), 83.3g of cholesterol and 30g of cholesterol-PEG1000 in absolute ethanol to obtain an organic phase. Add the organic phase to the water phase under the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com