Preparation method of long-acting hydrochloric ceftiofur injection
A technology of ceftiofur hydrochloride and injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as inconvenience in veterinary treatment and incompatibility in treating animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
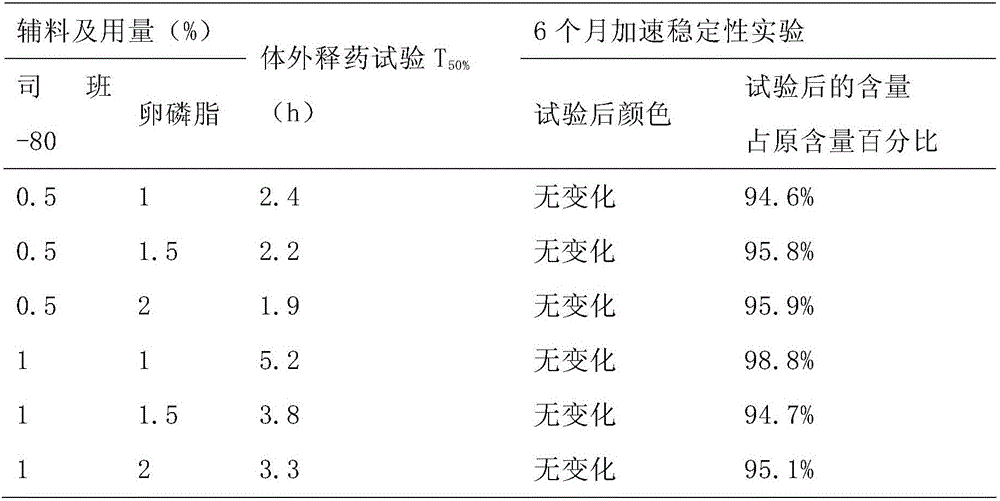
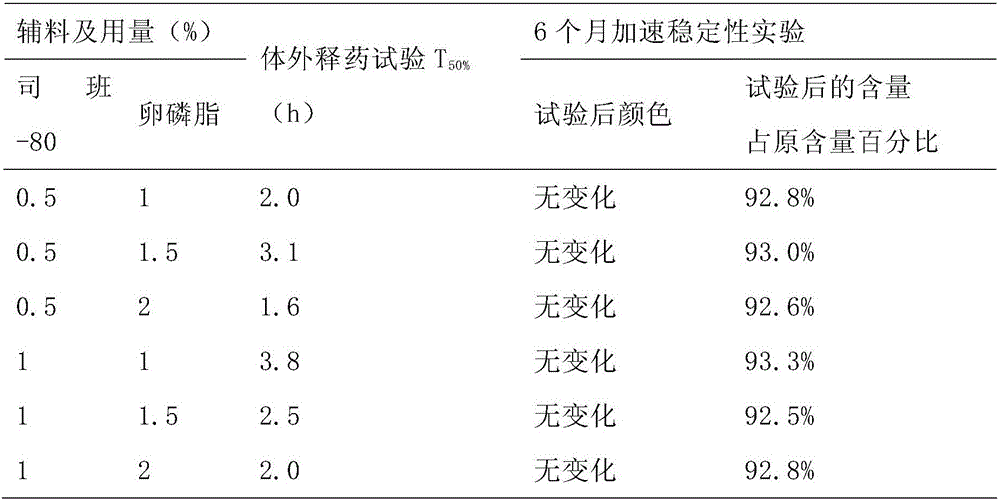
[0014] 1. Instruments and reagents
[0015] Colloid mill (Shanghai Donghua High Pressure Homogenizer Factory); Agilent 1100 high performance liquid chromatography; AG285 electronic analytical balance (Swiss Mettler Toledo Instrument Co., Ltd.); Ceftiofur reference substance (China Veterinary Drug Control Institute) , batch number: K0330702); Siban-80 (Shanghai Shenyu Pharmaceutical Chemical Co., Ltd.); lecithin (Shanghai Aikang Fine Chemical Co., Ltd.); soybean oil for injection (Tianyu Camellia Oil Development Co., Ltd.).
[0016] 2. Methods and results
[0017] 2.1 Prescription and process
[0018] 2.1.1 Prescription Composition
[0019] Ceftiofur hydrochloride 5kg, Span-80 (wetting agent) 0.5L, lecithin (suspending agent) 0.5L, soybean oil for injection (solvent) to 50L.
[0020] 2.1.2 Process
[0021] During the production process, all open surfaces in contact with the liquid medicine, such as preparation, filtration, and pipelines, should be treated as follows: prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com