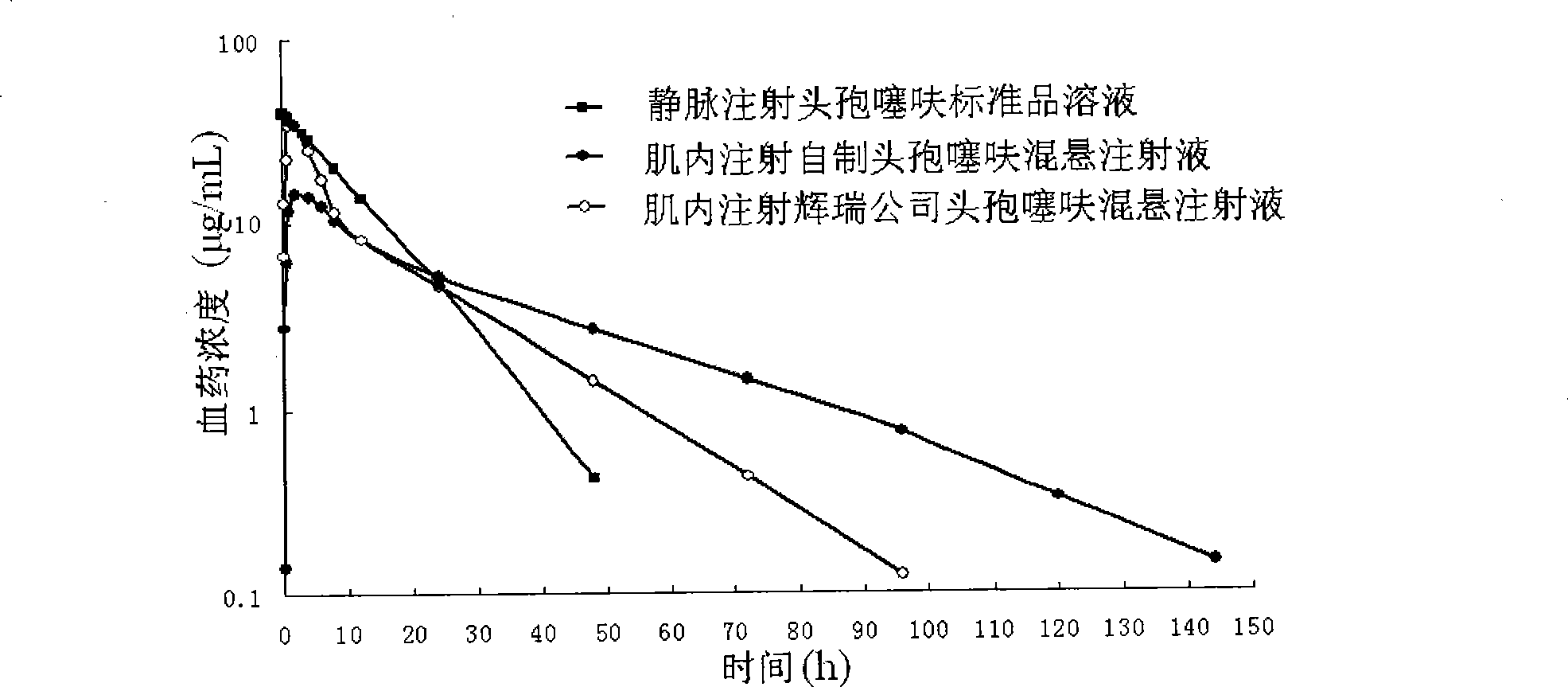
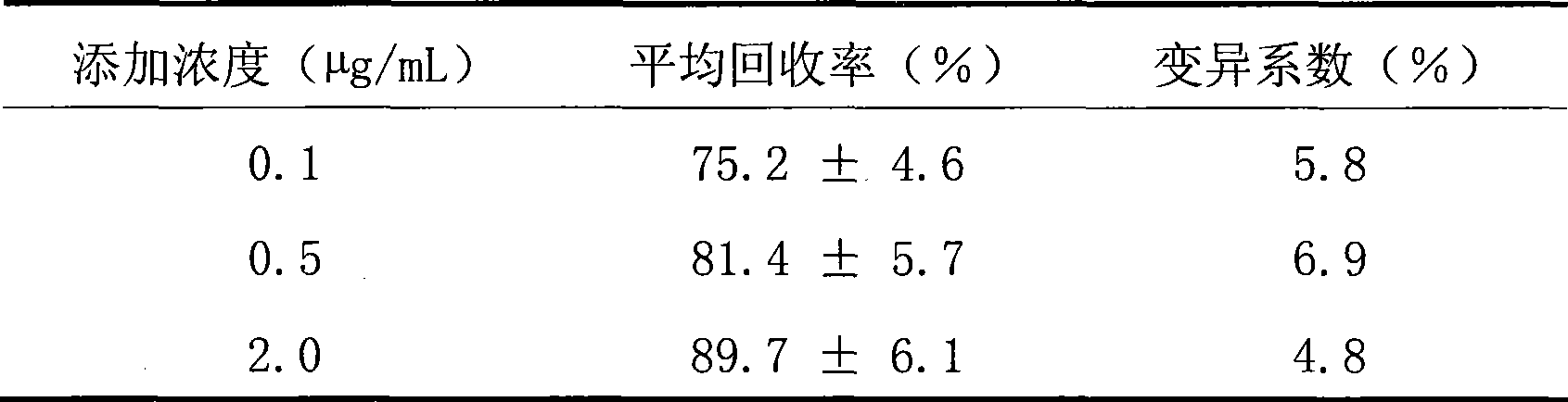
Ceftiofur long-acting injection and preparation method thereof
A technology of ceftiofur and ceftiofur hydrochloride, which is applied to medical preparations containing active ingredients, liquid delivery, pharmaceutical formulations, etc., can solve the problems of inconvenient clinical operation, poor safety, short drug action time, etc., and achieves reduction of frequent The effect of drug administration, saving manpower and material resources, and maintaining the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, preparation of ceftiofur long-acting injection
[0038] Prepare ceftiofur long-acting injection with the method of the present invention, and specific method comprises the following steps:
[0039] 1) Get ceftiofur hydrochloride aseptic powder and antioxidant (alpha-tocopherol), and use an ultrafine pulverizer to obtain ceftiofur hydrochloride aseptic micropowder with a particle size less than 25 microns through ultrafine pulverization in the aseptic operation room , α-tocopherol sterile micropowder;
[0040] 2) get suspending agent (aluminum stearate), cross 120 mesh medicine sieves, obtain aluminum stearate superfine powder;
[0041] 3) After the soybean oil for injection is sterilized at a high temperature of 150-160 degrees Celsius for 2 hours (2-3 hours are acceptable), it is placed at room temperature for use;
[0042] 4) Add 10 g of the ultrafine aluminum stearate powder obtained in step 2) to 900 mL of sterilized soybean oil for injection in step...
Embodiment 2
[0045] Embodiment 2, preparation of ceftiofur long-acting injection
[0046] Prepare ceftiofur long-acting injection with the method of the present invention, and specific method comprises the following steps:
[0047] 1) Get ceftiofur hydrochloride aseptic powder and antioxidant (alpha-tocopherol), and use an ultrafine pulverizer to obtain ceftiofur hydrochloride aseptic micropowder with a particle size less than 25 microns through ultrafine pulverization in the aseptic operation room , α-tocopherol sterile micropowder;
[0048] 2) get suspending agent (aluminum stearate), cross 120 mesh medicine sieves, obtain aluminum stearate superfine powder;
[0049] 3) After the soybean oil for injection is sterilized at a high temperature of 150-160 degrees Celsius for 2 hours (2-3 hours are acceptable), it is placed at room temperature for use;
[0050] 4) Add 15 g of the ultrafine aluminum stearate powder obtained in step 2) to 900 mL of sterilized soybean oil for injection in step...
Embodiment 3
[0053] Embodiment 3, preparation of ceftiofur long-acting injection
[0054] Prepare ceftiofur long-acting injection with the method of the present invention, and specific method comprises the following steps:
[0055] 1) Get ceftiofur hydrochloride aseptic powder and antioxidant (alpha-tocopherol), and use an ultrafine pulverizer to obtain ceftiofur hydrochloride aseptic micropowder with a particle size less than 25 microns through ultrafine pulverization in the aseptic operation room , α-tocopherol sterile micropowder;
[0056] 2) Take the suspending agent (polyvinylpyrrolidone) and pass through a 120-mesh drug sieve to obtain a very fine polyvinylpyrrolidone powder;
[0057] 3) Glyceryl triacetate for injection is sterilized at 150-160 degrees Celsius for 2 hours (2-3 hours are acceptable), and placed at room temperature for later use;
[0058] 4) Add 20 g of polyvinylpyrrolidone ultrafine powder obtained in step 2) to 900 mL of sterilized triacetin for injection in step ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com