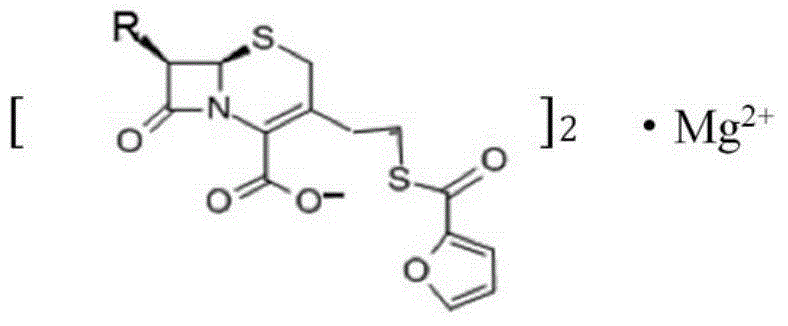
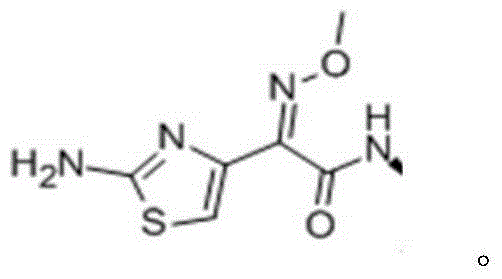
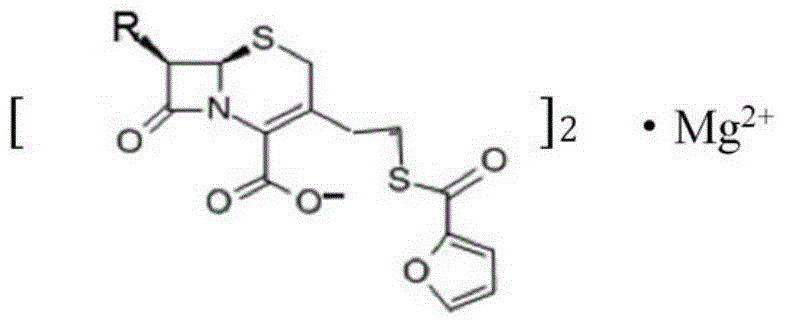
Magnesium ceftiofur as well as preparation method and using method thereof
A technology of ceftiofur and ceftiofur hydrochloride, which is applied in pharmaceutical formulas, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problem of slow metabolism of benzylethylenediamine ceftiofur and achieve effective blood drug concentration , low technical threshold and other issues, to achieve the effect of prolonging the drug action time, improving drug value and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0028] The preparation method of Ceftiofur Magnesium of the present invention comprises the following steps:
[0029] (1) Add 1 mol (560 g) of ceftiofur hydrochloride into 2240 ml of tetrahydrofuran and stir thoroughly; then add 224 ml of water for injection and fully stir to obtain solution A;
[0030] (2) in solution A, add 0.5mol (20.15g) gradually and the magnesia superfine powder that particle size is 150 microns, stir while adding, react to obtain solution B, promptly contain product ceftiofur magnesium in solution B;
[0031] (3) filter solution B, get upper layer solution C 2000mL; add the carbon tetrachloride that is equal to the volume of upper layer solution C and fully extract solution C, obtain extraction solution 2000mL;
[0032] (4) Evaporate the extract to dryness at 37°C and 10kPa to obtain a white powder; wash the white powder with 1500 mL of acetone three times, 500 mL each time, and evaporate to dryness to obtain 550 g of ceftiofur magnesium.
[0033] The ...
Embodiment 2
[0061]Metabolic kinetics experiment of ceftiofur magnesium in cows
[0062] 1. Injection Preparation
[0063] Prepare the obtained ceftiofur magnesium (W / W, 10%) with soybean oil for injection (W / W, 20%), propylene glycol (W / W, 11%), PEG-200 (W / W, 9%), Lecithin (W / W, 7%), Ethyl Oleate (W / W, 13%), Cyclodextrin (W / W, 9%), Water for Injection (W / W, 21%) After mixing, an injection solution with a mass fraction of 10% is prepared, and the injection solution is a suspension.
[0064] 2. Materials and Reagents
[0065] High-performance liquid chromatography, adult Holstein lactating cows, disposable syringes, blood collection devices, 10% ceftiofur magnesium injection, and imported ceftiofur injection (Yizuda) were used as control drugs.
[0066] 3. Experimental operation
[0067] Take 20 adult Holstein lactating cows that have not been treated with any antibiotics within one month. After collecting 5ml blood samples, divide them into 2 groups, 10 cows in each group; According t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com