Preparation method of hydrochloric acid ceftiofur
A technology of ceftiofur hydrochloride and furyl carbonyl, which is applied in the field of chemical synthesis of veterinary chemical raw materials, can solve the problems of long cycle, inconvenient large-scale production, cumbersome operation, etc., and achieve less impurities, less toxic and side effects, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
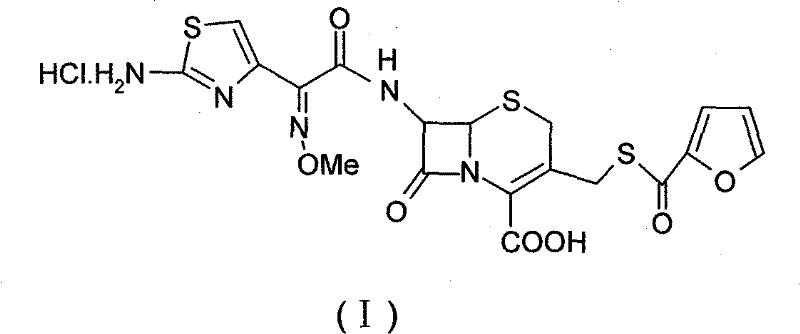
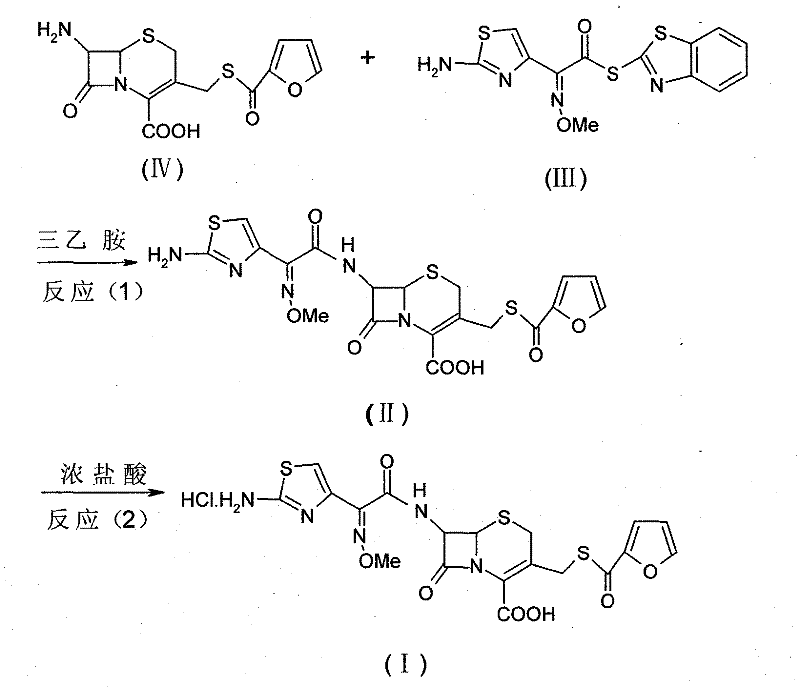
[0038] Example 1 (6R, 7R)-7-[2-(2-aminothiazol-4-yl)-(Z)-2-(methoxyimino)acetamido]-3-[(2-furancarbonyl ) Synthesis of thiomethyl]-3-cephem-4-carboxylic acid (II).
[0039] In a 500ml three-necked reaction flask, add 198ml of acetone, cool down in an ice bath, stir for 30 minutes, add 5.28g of cefuroxime, 10.2g of AE-active ester and 1.2g of antioxidant BHT in batches, stir for 25 minutes, and then add triethyl ether dropwise 10.6ml of amine was added dropwise in 30 minutes, kept in an ice bath for 6 hours, and the reaction was complete. Raise the temperature to 20-35°C, add 198ml of acetone to dilute, add 19.0ml of water, then add 5.0g of activated carbon, stir for 45 minutes to decolorize, and remove the activated carbon by suction filtration to obtain (6R,7R)-7-[2-( 2-aminothiazol-4-yl)-(Z)-2-(methoxyimino)acetamido]-3-[(2-furancarbonyl)thiomethyl]-3-cephem-4-carboxylic acid (II), namely ceftiofur free acid solution.
Embodiment 2
[0040] The synthesis of embodiment 2 ceftiofur hydrochloride:
[0041] Add the ceftiofur free acid solution prepared in Example 1 into a 1000ml three-necked reaction flask, stir, add 5ml of concentrated hydrochloric acid (37.7%) drop by drop, a large amount of crystals are precipitated after the addition, stir for 1 hour, and place at room temperature overnight to grow crystals . Suction filtration to obtain a wet product of ceftiofur hydrochloride, and drying under reduced pressure at 30-35°C for 24 hours to obtain 7.2 g of ceftiofur hydrochloride white to off-white crystalline powder with a purity of 99.7%.
Embodiment 3
[0042] The synthesis of embodiment 3 ceftiofur hydrochloride:
[0043] In a 1000ml three-necked reaction flask, add 17.28g of cefurofuric acid and 432ml of tetrahydrofuran, stir for 30 minutes, add 21.32g of AE-active ester in batches, add 2.33g of antioxidant BHT, and cool in an ice bath for 1 hour. 25.3 ml of triethylamine was added, and the addition was completed in 30 minutes. Keep the reaction in an ice bath for 6 hours. After the reaction is complete, add 21ml of water, slowly warm up to room temperature, then add an appropriate amount of concentrated hydrochloric acid to adjust the pH value to 1.0, stir for 1 hour, crystals gradually precipitate, and place at room temperature overnight to grow crystals. Suction filtration to obtain ceftiofur hydrochloride wet product, dried under reduced pressure at 30-35°C for 24 hours, ceftiofur hydrochloride white to off-white crystalline powder 25.3g, purity 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com