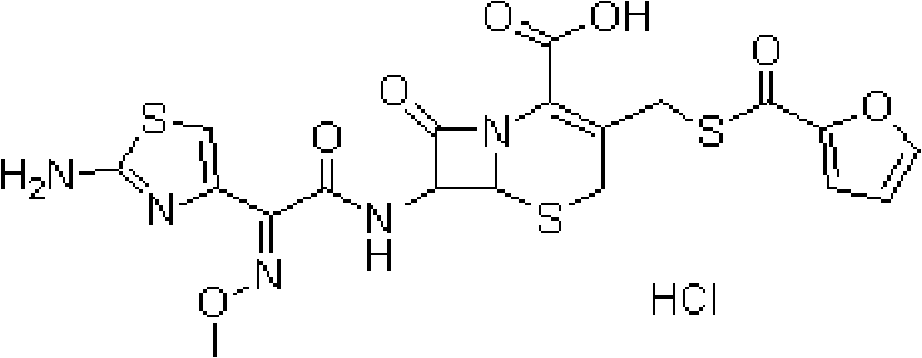
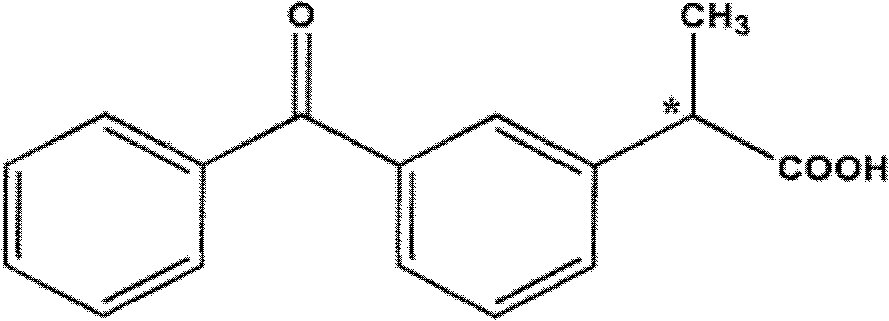
Formulations comprising ceftiofur and ketoprofen or ceftiofur and benzyl alcohol
A technology of ceftiofur, preparation, applied in the field of preparation containing ceftiofur and ketoprofen or ceftiofur and benzyl alcohol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Effect of benzyl alcohol on physical properties of Ceftiofur HCl oily suspension.
[0136] Table 1
[0137] Laboratory batches were prepared with and without 1% benzyl alcohol.
[0138]
[0139] The following observations were made: formulations with benzyl alcohol separated greater on standing in 100 mL laboratory rectangular vials compared to batches without benzyl alcohol. This result is surprising and unexpected.
[0140] Initial observations revealed day 4 separation in 100 mL laboratory rectangular vials. The sedimentation volume (F) is the equilibrium volume of the sediment (V u ) and the total volume of the suspension (V 0 )The ratio. Thus, F=V u / V 0 . The F-value (typically in the range of approximately 0 to 1) increases as the volume of suspension occupied by the precipitate appears to increase. In a system where F=0.75, for example, 75% of the total volume in the vessel is apparently occupied by loose, porous flocs forming the precipitate. When ...
Embodiment 2
[0167] A batch of ceftiofur containing benzyl alcohol is Comparison of RTUs
[0168] Ceftiofur formulations (Lots E-H, respectively) were prepared with 0, 1.0, 2.0, and 3.0% benzyl alcohol, and their physical properties were comparable to those prepared by Pfizer RTU sterile suspension (ceftiofur hydrochloride) was compared, which did not contain benzyl alcohol. The settling volume standing in a 100 mL graduated cylinder was measured. Sedimentation volume (%) = [(volume of sediment x 100) / (total volume)] and the results are summarized in Table 8.
[0169] Table 8
[0170] Sedimentation volume (%) = (volume of sediment x 100) / total volume)
[0171]
[0172] NR = not recorded
[0173] The viscosity was measured in a Brookfield LV 2 at 100 rpm and the results are summarized in Table 9.
[0174] Table 9
[0175] Viscosity
[0176] lot E
Lot F
batch G
batch H
0%
1%
2%
3%
129cps
136cps
...
Embodiment 3
[0189] Development of a combination Ceftiofur HCl and Ketoprofen oily suspension
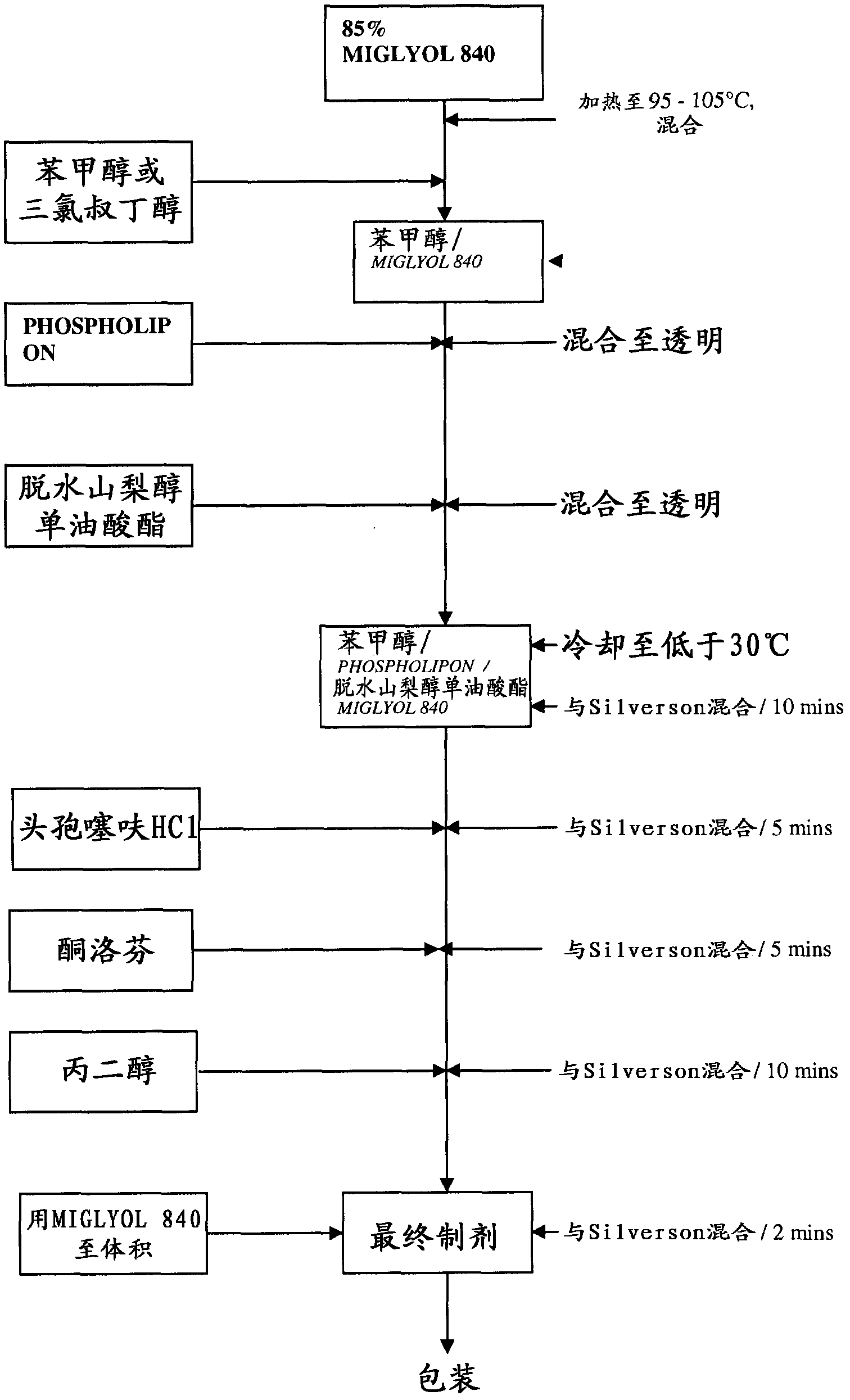
[0190] The following outlines the development of a stable, easily resuspendable combination Ceftiofur HCl and Ketoprofen oily suspension for injection comprising 5.0% w / v ceftiofur and 15.0% ceftiofur w / v ketoprofen suitable for intramuscular and subcutaneous injection. The desired formulation contained 5.0% ceftiofur HCL and excipients; PHOSPHOLIPON 90H, sorbitan monooleate, propylene glycol and benzyl alcohol in a refined cottonseed oil vehicle. Attempt to incorporate ethanol as an excipient in the original ceftiofur HCL injection formulation.
[0191] Formulation 1
[0192] Intent: To compare the addition of various concentrations of benzyl alcohol and ethanol. NB - Ketoprofen Free.
[0193] The sub-batches had the following amounts of benzyl alcohol and ethanol:
[0194] 1: 15% benzyl alcohol / 0% ethanol
[0195] 2: 10% benzyl alcohol / 0% ethanol
[0196] 3: 0% benzyl alcohol / 0% ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com