Ceftiofur hydroxypropyl-beta-cyclodextrin inclusion compound and preparation method thereof
A technology of cyclodextrin inclusion complex and thifuryl hydroxypropyl is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Solubility, improving bioavailability, increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The preferred embodiments of the present invention will be described below in conjunction with the accompanying drawings. It should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
[0022] 1. Study on Phase Solubility Method
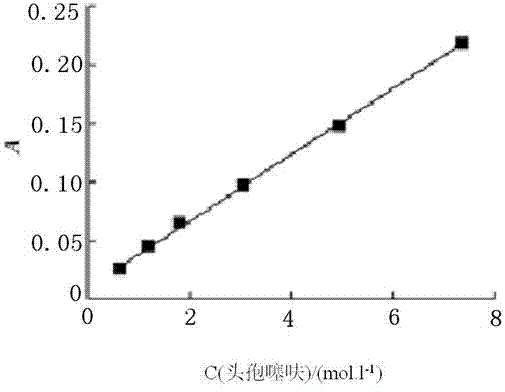
[0023] In the low concentration system, the dilution effect makes the effect of hydroxypropyl-β-cyclodextrin (HP-β-CD) on the UV absorption of ceftiofur negligible. The obtained standard curve is Y=0.0279X+0.0118 (R 2 =0.9997) ( figure 1 ).
[0024] According to the results of the standard curve, when the concentration of HP-β-CD is 0, the intrinsic solubility of ceftiofur is equal to 4.70×10 -7 mol / L.
[0025] 2. Optimization of inclusion conditions
[0026] 1. The influence of different proportions of raw materials on inclusion
[0027] When ceftiofur and hydroxypropyl-β-cyclodextrin (HP-β-CD) were mixed at a temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com