Compound long-effect injection containing ceftiofur and meloxicam and preparation method thereof
A technology of ceftiofur and meloxicam, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, oil/fat/wax non-active ingredients, etc., can solve the restrictions on the development and application of compound injections, and the content of substances exceeds the standard , poor stability of ceftiofur, etc., to achieve the effect of enhancing animal compliance, promoting outcome and prognosis, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
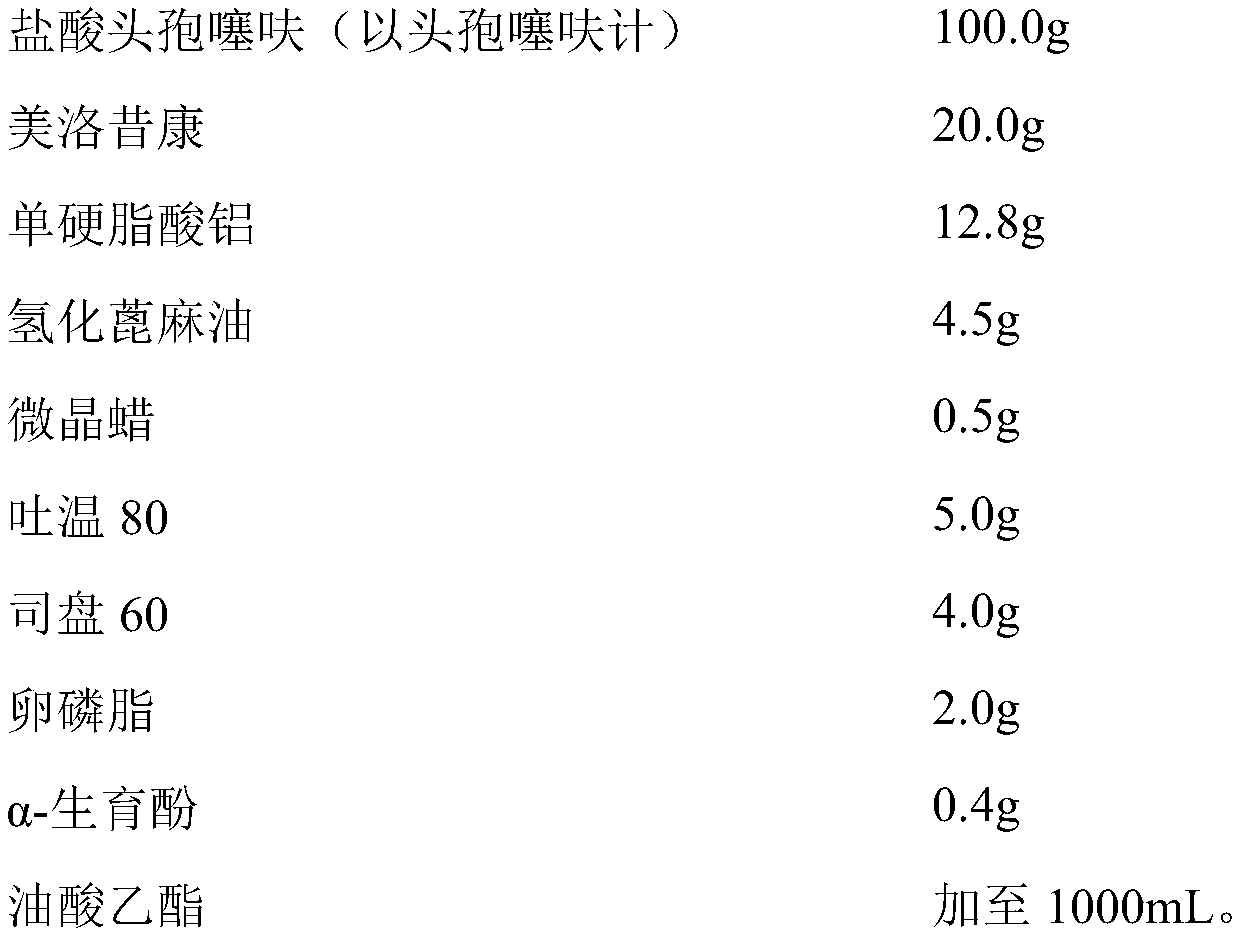
[0039] prescription
[0040]
[0041] Preparation method: Take 1000mL of ethyl oleate, heat it to 170°C for 1 hour, then cool down to 120°C, take out 600mL, and cool down the rest of ethyl oleate to room temperature for constant volume. Add aluminum monostearate and hydrogenated castor oil to 600mL of ethyl oleate at a temperature of 120°C, and maintain at 120°C for 1.5 hours to completely gel the aluminum monostearate and hydrogenated castor oil; let cool to below 40°C, add Microcrystalline wax, Tween 80, Span 60, lecithin, α-tocopherol, ceftiofur hydrochloride (particle size D90≤5μm), meloxicam (particle size D90≤5μm), high pressure homogenization (pressure 20000psi) Treat for 1.5h; add ethyl oleate cooled to room temperature and make the volume to 1000mL. Check the particle size, related substances and content; subpackage after passing the test.
[0042] The in vitro performance evaluation of the prepared compound long-acting injection containing ceftiofur and meloxica...
Embodiment 2
[0050] prescription
[0051]
[0052] Preparation method: Take 1000mL of ethyl oleate, heat it to 160°C for 1 hour, then cool down to 130°C, take out 600mL, and cool down the remaining ethyl oleate to room temperature for constant volume. Add aluminum monostearate and hydrogenated castor oil to 600mL of ethyl oleate at a temperature of 130°C, and maintain at 130°C for 1 hour to completely gel the aluminum monostearate and hydrogenated castor oil; let cool to below 40°C, add spit Wen 80, Span 40, lecithin, propyl gallate, ceftiofur hydrochloride (particle size D90≤5μm), meloxicam (particle size D90≤5μm), high-speed shearing instrument (speed 25000 rpm) for 1.5 h; Add ethyl oleate cooled to room temperature and make the volume to 1000 mL. Detect the particle size, related substances and content; subpackage after passing the test, and obtain the compound long-acting injection containing ceftiofur and meloxicam, and evaluate its in vitro performance, including character, parti...
Embodiment 3
[0054] prescription
[0055]
[0056]
[0057] Preparation method: Take 1000mL of isopropyl myristate, heat to 160°C for 1 hour, then cool down to 120°C, take out 600mL, and cool the remaining isopropyl myristate to room temperature for constant volume. Add hydrogenated castor oil to 600mL of isopropyl myristate at 120°C, maintain at 120°C for 1 hour to make it completely gelled; let cool to below 40°C, add microcrystalline wax, Tween 80, Span 60, egg Phospholipids, propyl gallate, ceftiofur hydrochloride (particle size D90≤5μm), meloxicam (particle size D90≤5μm), high pressure homogenization (20000psi) treatment for 2h; add isopropyl myristate cooled to room temperature Capacity to 1000mL. Detect the particle size, related substances and content; subpackage after passing the test to obtain the compound long-acting injection containing ceftiofur and meloxicam, and evaluate its in vitro performance, including character, particle size, 3h sedimentation volume ratio, and r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com