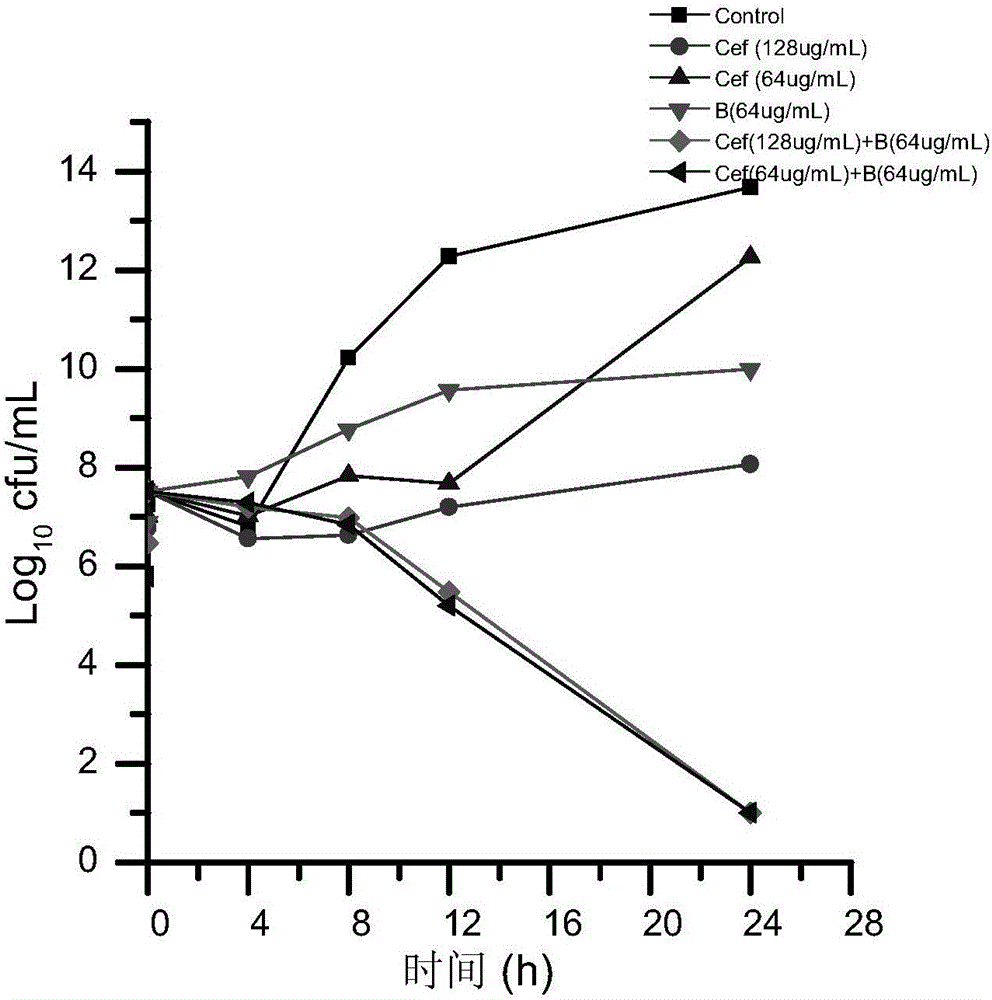
Veterinary suspension containing ceftiofur and baicalein and preparing method thereof
A technology of ceftiofur and suspension, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, liquid delivery, etc., to achieve the effects of simple production process, cost reduction, and alleviation of drug resistance problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
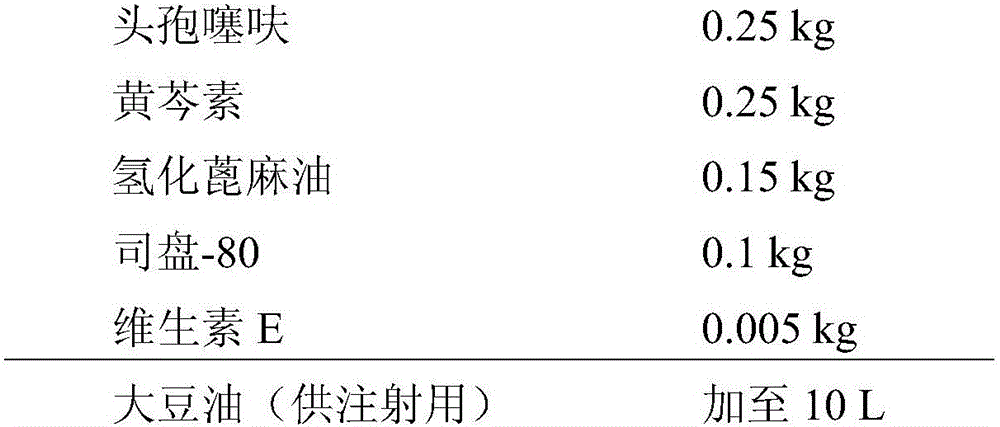
[0087] Embodiment 1, preparation contains the compound suspension of ceftiofur baicalein
[0088] Its formula is as follows:
[0089]
[0090] The specific preparation method is as follows:
[0091] 1) Take the hydrogenated castor oil of the formula and add it to 0.5L soybean oil, heat and stir to dissolve it completely to obtain liquid A, and set aside;
[0092] 2) Pour 8L of soybean oil into the colloid mill, start the colloid mill, slowly add the prescribed amount of ceftiofur, grind for about 5 minutes, slowly add Span-80 and liquid A, and continue grinding for about 15 minutes.
[0093] 3) Slowly add the prescribed amount of baicalein and vitamin E, add soybean oil to 10L, and continue grinding for 15 minutes.
[0094] 4) Check the fineness of the particles: no less than 90% of the particles with a particle size of 15 μm or less, no less than 95% of the particles with a particle size of 20 μm or less, and no particles exceeding 50 μm. If the result meets the requirem...
Embodiment 2
[0095] Embodiment 2, preparation contains the compound suspension of ceftiofur baicalein
[0096] Its formula is as follows:
[0097]
[0098] The specific preparation method is as follows:
[0099] 1) Take the hydrogenated castor oil of the formula and add it to 0.5L soybean oil, heat and stir to dissolve it completely to obtain liquid A, and set aside;
[0100] 2) Pour 8L of soybean oil into the colloid mill, start the colloid mill, slowly add the prescribed amount of ceftiofur, grind for about 5 minutes, slowly add Span-80 and liquid A, and continue grinding for about 15 minutes.
[0101] 3) Slowly add the prescribed amount of baicalein and vitamin E, add soybean oil to 10L, and continue grinding for 15 minutes.
[0102] 4) Check the fineness of the particles: no less than 90% of the particles with a particle size of 15 μm or less, no less than 95% of the particles with a particle size of 20 μm or less, and no particles exceeding 50 μm. If the result meets the requirem...
Embodiment 3
[0103] Embodiment 3, preparation contains the compound suspension of ceftiofur baicalein
[0104] Its formula is as follows:
[0105]
[0106] The specific preparation method is as follows:
[0107] 1) Add hydrogenated castor oil and propyl gallate in 0.5L of glycerol triacetate in the prescribed amount, heat and stir to completely dissolve to obtain liquid A, and set aside;
[0108] 2) Take 8L triacetin and pour it into the colloid mill, start the colloid mill, slowly add the prescribed amount of ceftiofur, grind for about 5 minutes, slowly add Tween-80 and liquid A, and continue grinding for about 15 minutes.
[0109] 3) Slowly add the prescribed amount of baicalein, add soybean oil to 10L, and continue grinding for 15 minutes.
[0110] 4) Check the fineness of the particles: no less than 90% of the particles with a particle size of 15 μm or less, no less than 95% of the particles with a particle size of 20 μm or less, and no particles exceeding 50 μm. If the result mee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com