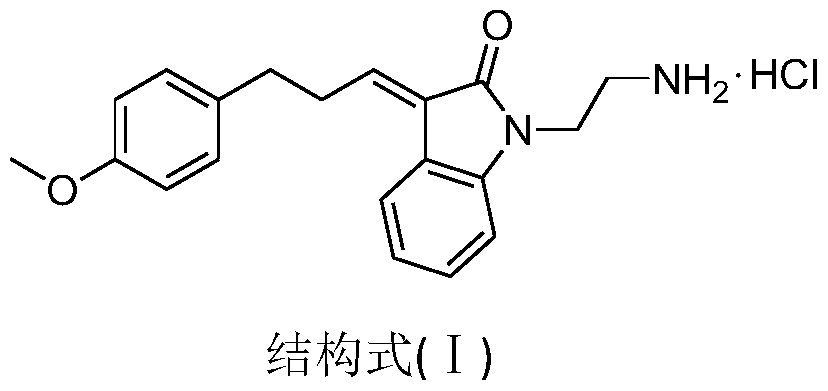
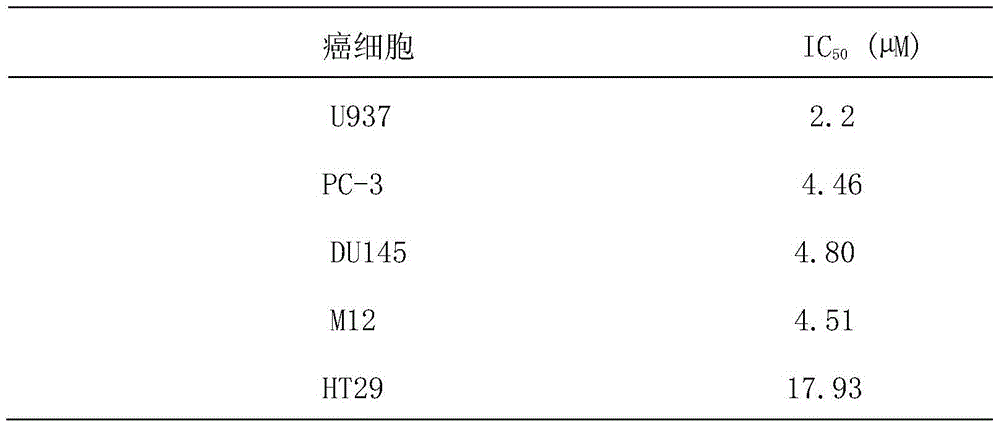
A dual channel inhibitor targeting raf/mek/erk and pi3k/akt
An inhibitor and dual-channel technology, applied in medical preparations containing active ingredients, drug combinations, organic chemistry, etc., can solve the problems of undiscovered small molecular substances, increased drug dosage, and increased human toxicity, and achieve The effect of reducing the probability of drug resistance, reducing toxicity, and increasing potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
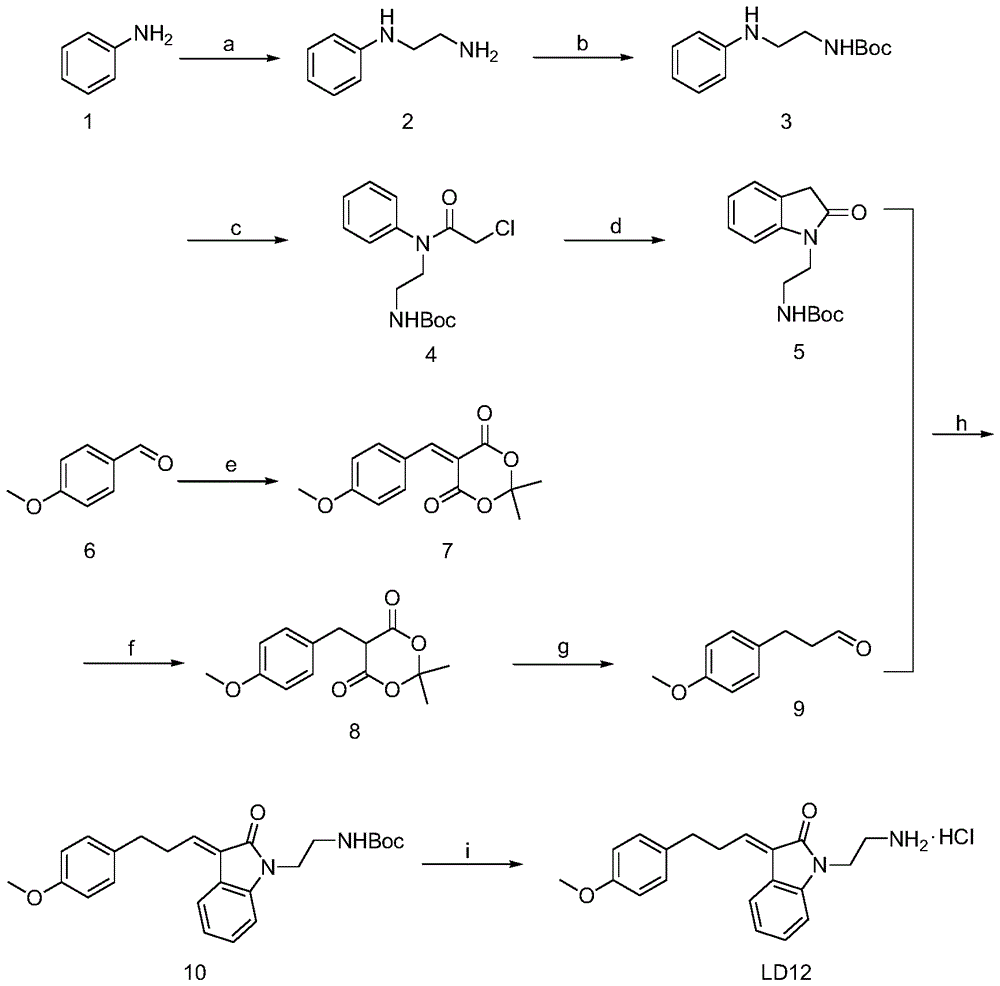
Embodiment 1
[0036] 1). The synthesis of N-phenylethylenediamine (2):
[0037] 2-Bromoethylamine hydrobromide (0.1 mmol) and aniline (1) (0.2 mmol) were added to 40 ml of toluene, heated to reflux for 16 hours, and cooled. Add 60ml of water and 20ml of 50% KOH aqueous solution, and let stand to separate layers. The aqueous phase was washed with saturated NaCl and extracted three times with dichloromethane. Combined organic phases were washed with saturated NaCl, anhydrous Na 2 SO 4 Dry, filter and evaporate to dryness. The pure compound (2) was obtained by column chromatography with a yield of 60%. 1 H(400MHz,MeOH):δ7.10-7.08(t,J=3.4Hz,2H),6.67-6.60(m,3H),3.23-3.20(t,J=6.08Hz,2H),2.90-2.87( t, J=6.24Hz, 2H).
[0038] 2). Synthesis of N-tert-butoxycarbonyl-N'-phenylethylenediamine (3):
[0039] N-phenylethylenediamine (2) (1.0 mmol) and di-tert-butyl carbonate (1.2 mmol) were dissolved in 15 ml of methanol, stirred for 4 hours, and concentrated under reduced pressure to obtain a brown ...
Embodiment 2
[0054] 1). The synthesis of N-phenylethylenediamine (2):
[0055] 2-Bromoethylamine hydrobromide (0.1 mmol) and aniline (1) (0.25 mmol) were added to 40 ml of toluene, heated to reflux for 18 hours, and cooled. Add 60ml of water and 20ml of 50% KOH aqueous solution, and let stand to separate layers. The aqueous phase was washed with saturated NaCl and extracted three times with dichloromethane. Combined organic phases were washed with saturated NaCl, anhydrous Na 2 SO 4 Dry, filter and evaporate to dryness. The pure compound (2) was obtained by column chromatography with a yield of 62%.
[0056] 2). Synthesis of N-tert-butoxycarbonyl-N'-phenylethylenediamine (3):
[0057] N-phenylethylenediamine (2) (1.0 mmol) and di-tert-butyl carbonate (1.5 mmol) were dissolved in 15 ml of methanol, stirred for 5 hours, and concentrated under reduced pressure to obtain a brown oil. The pure white solid compound (3) was obtained by column chromatography with a yield of 90%.
[0058] 3)...
Embodiment 3
[0070] 1). The synthesis of N-phenylethylenediamine (2):
[0071] Add 2-bromoethylamine hydrobromide (0.1 mmol) and aniline (1) (0.15 mmol) into 40 ml of toluene, heat to reflux for 14 hours, and cool. Add 60ml of water and 20ml of 50% KOH aqueous solution, and let stand to separate layers. The aqueous phase was washed with saturated NaCl and extracted three times with dichloromethane. Combined organic phases were washed with saturated NaCl, anhydrous Na 2 SO 4 Dry, filter and evaporate to dryness. The pure compound (2) was obtained by column chromatography with a yield of 58%.
[0072] 2). Synthesis of N-tert-butoxycarbonyl-N'-phenylethylenediamine (3):
[0073] N-phenylethylenediamine (2) (1.0 mmol) and di-tert-butyl carbonate (1.0 mmol) were dissolved in 15 ml of methanol, stirred for 3 hours, and concentrated under reduced pressure to obtain a brown oil. The pure white solid compound (3) was obtained by column chromatography with a yield of 86%.
[0074] 3). Synthes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com