Method of preparing ceftiofur
A technology of ceftiofur and cefuroxime, which is applied in the direction of organic chemistry, etc., can solve the problems of increased drug resistance of pathogenic bacteria, decreased drug efficacy, and increased use concentration of antibiotics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 2
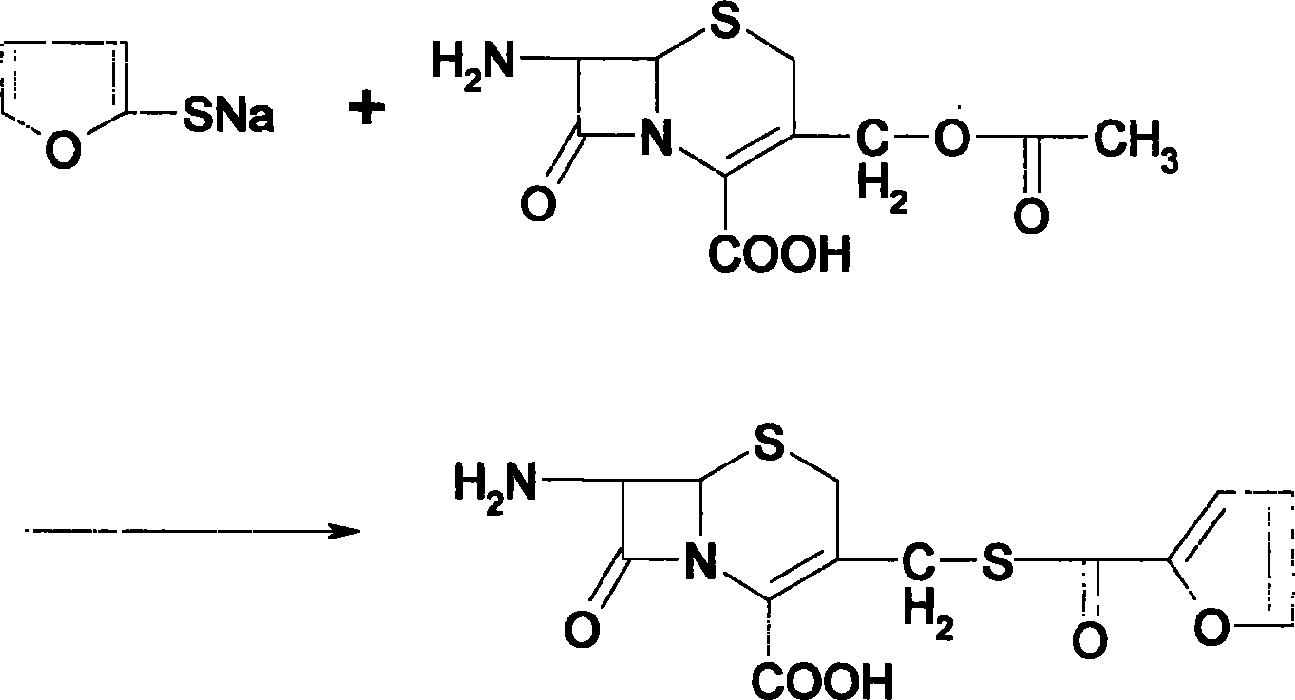
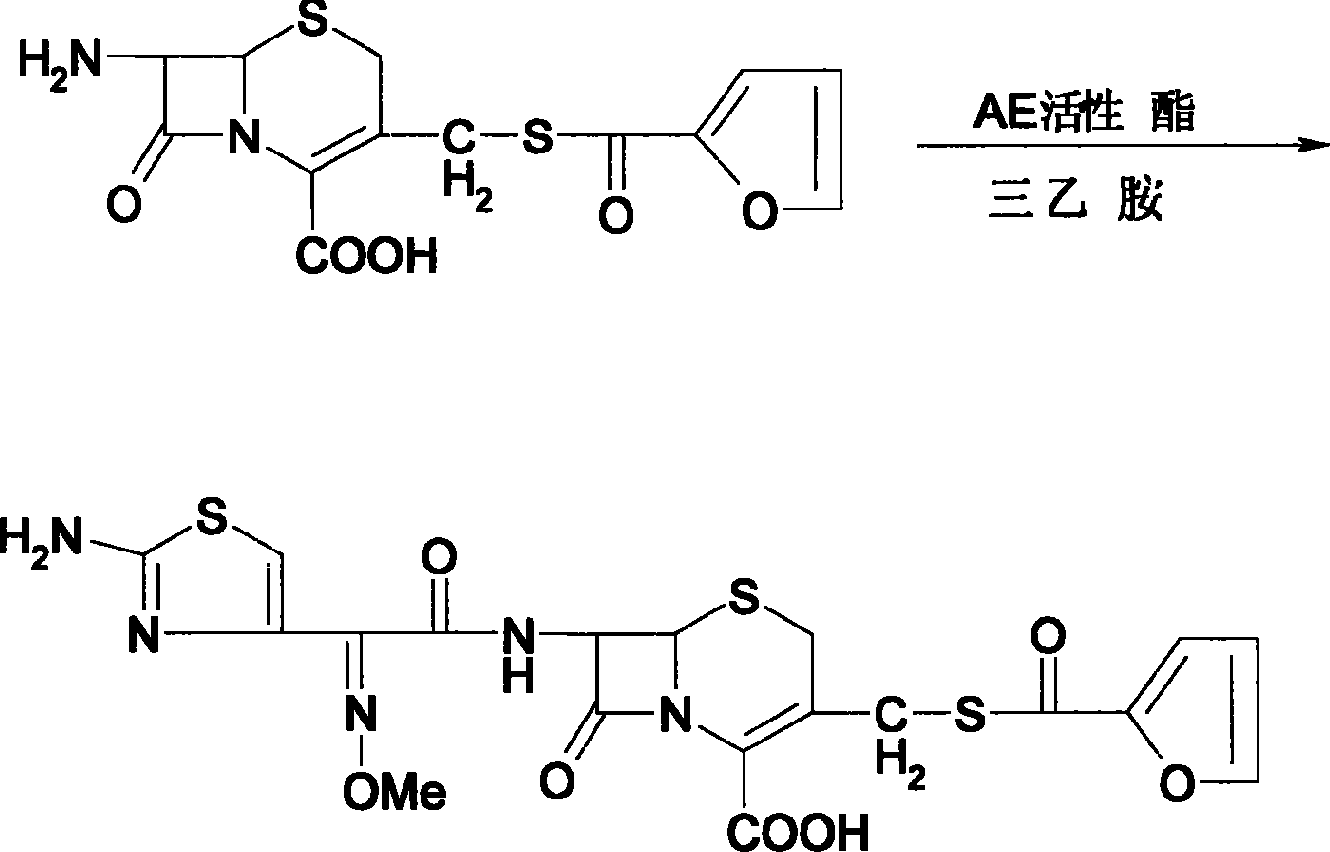
[0019] Add 40g of sodium hydrosulfide to 400g of purified water and stir to completely dissolve, add dropwise sugar acyl chloride and 40% sodium hydroxide solution to keep the pH value at 9-10, and then adjust the pH value to 4-5 with phosphoric acid. After keeping for 1 hour, add sodium hydroxide solution to adjust the pH to 8-9, extract twice with ethyl acetate, add 7-ACA62g to the water layer for reaction, cool, filter, wash with water, wash with acetone, and dry at room temperature to obtain cefuroxime 71.1g; Add 1000ml of dichloromethane, 71.1g of cefuroxime, 80g of AE active ester, dropwise add 72g of triethylamine, keep at 5-10°C for 3 hours, extract with purified water, combine the water layers, add 8g of activated carbon, and stir After 1 hour, filter by rejection, adjust the pH value of the filtrate to 4-5 with 10% hydrochloric acid solution, and obtain 73.3 g of ceftiofur by rejection by filtration. (Total yield 61.5%, effective content 98.9%).
specific Embodiment approach 3
[0020] Add 40g of sodium hydrosulfide to 400g of purified water and stir to completely dissolve, add dropwise sugar acyl chloride and 40% sodium hydroxide solution to keep the pH value at 9-10, and then adjust the pH value to 4-5 with phosphoric acid. After keeping for 1 hour, add sodium hydroxide solution to adjust the pH to 8-9, extract twice with dichloromethane, add 61 g of 7-ACA to the aqueous layer, react, shake off, wash with water, wash with chloroform, and dry at room temperature to obtain cefuroxime 72.6g; Add 500g of dichloromethane, 72.6g of cefuroxime, 80g of AE active ester, dropwise add 72g of triethylamine, keep the reaction at 5-10°C for 3.5 hours, extract with purified water, combine the water layers, add 10g of activated carbon, and stir After 1 hour, shake off, adjust the pH value of the filtrate to 4-5 with 10% hydrochloric acid solution, and shake off to obtain 75.0 g of ceftiofur. (Total yield 63.9%, effective content 99.3%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com