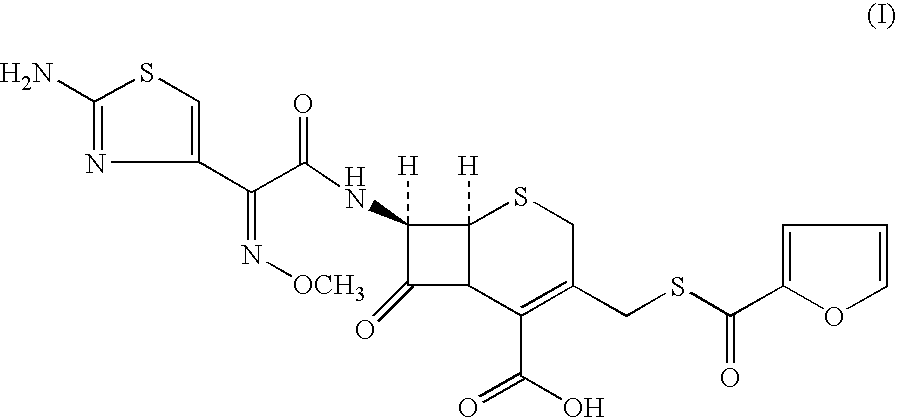
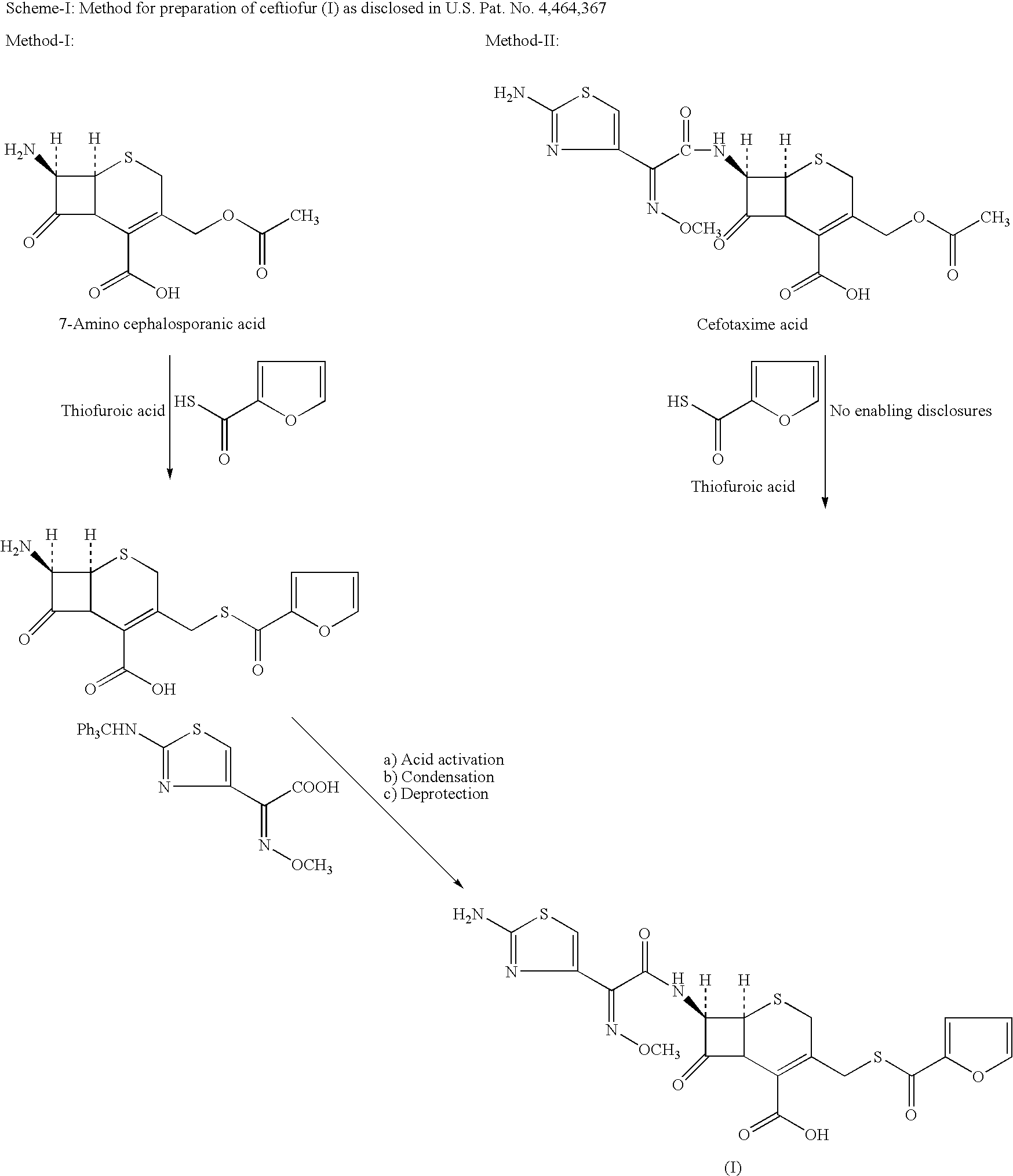
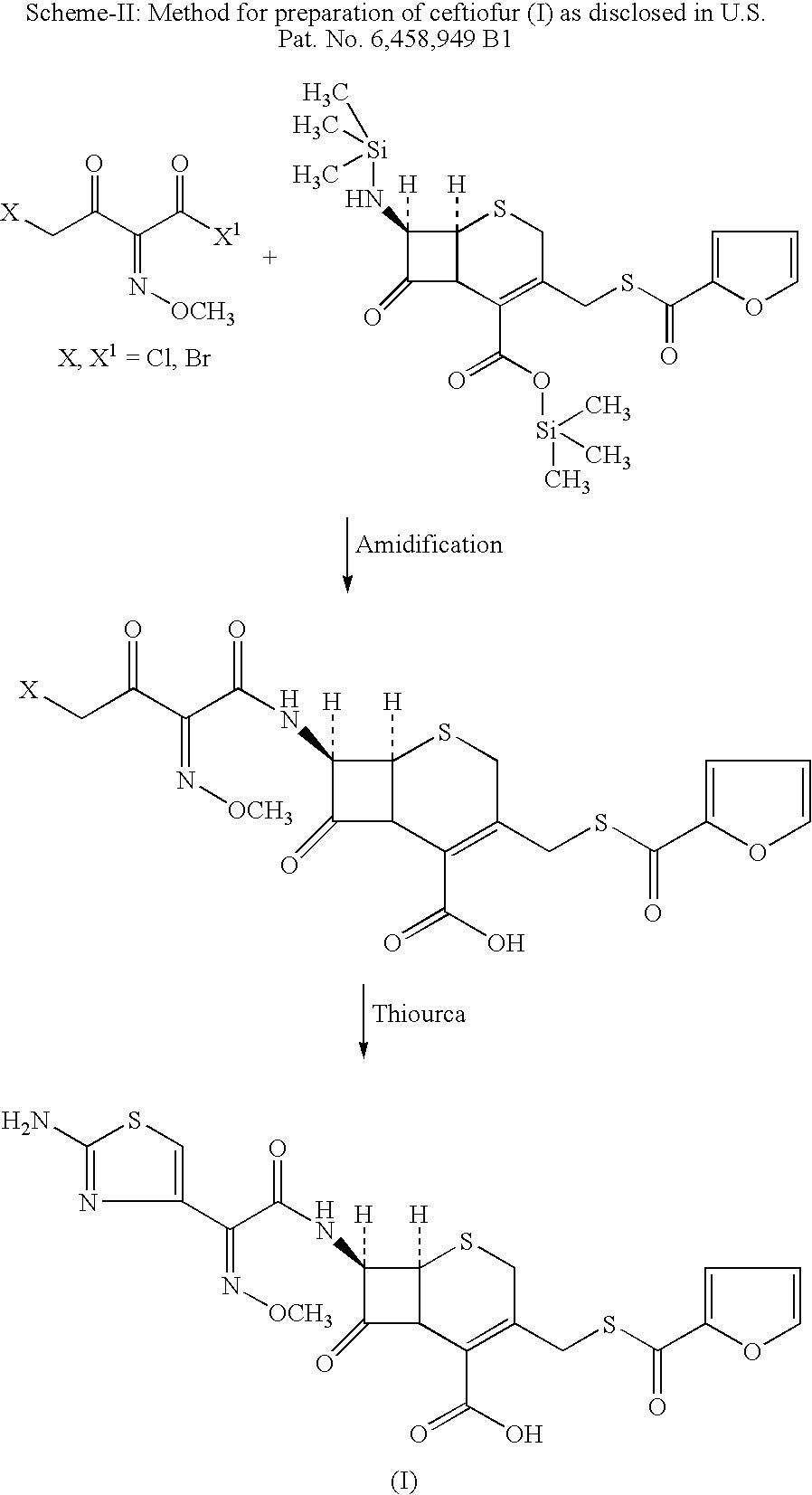
Method for manufacture of ceftiofur
a technology of ceftiofur and ceftiofur, which is applied in the field of improved methods for manufacturing ceftiofur, can solve the problems of difficult removal of ceftiofur, method described, and dicyclohexyl urea, and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (6R, 7R)-7-[[(2Z)-(2-amino-4-thiazolyl) (methoxyimino) acetyl]amino]-3-[[(2-furanylcarbonyl) thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (I). (Mixture of Water Immiscible Solvent and Water in the Presence of Triethyl Amine as Base)
[0106]7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid(III) (100 gms; 0.294 moles) was added to dichloromethane (1000 ml). The reaction mixture was cooled to 0° C. and triethyl amine (53.57 gms; 0.529 moles) was added at 0-5° C. in 60 minutes. [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester (II) (123.5 gms; 0.353 moles) was added and agitated for 15 minutes. Water (25.0 ml) was added to the mixture and agitated at 5-7° C. The reaction was monitored by HPLC and the mixture stirred till compound (III) was less than 1.0% on HPLC. The reaction mixture was worked up by adding water (700 ml) and stirred for 15 minutes at 10-15° C. The aqueous layer was separated an...
example 2
Preparation of (6R, 7R)-7-[[(2Z)-(2-amino-4-thiazolyl) (methoxyimino)acetyl]amino]-3-[[(2-furanylcarbonyl) thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (I) (Water Immiscible Solvent and Water Miscible Solvent Without Water)
[0107]7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid (III) (5.0 gms; 0.0147 moles) was added to dichloromethane (50 ml). The reaction mixture was cooled to 0° C. and [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester (H) (5.4 gms; 0.0154 moles) was added to the mixture with agitation. Triethyl amine (2.67 gms; 0.0264 moles) was added to the mixture followed by methanol (2.5 ml). The reaction mixture was monitored by HPLC and stirred for 3.0 hours at 5±2° C.; the reaction was not going to completion as 2.5% was remaining unreacted even after stirring further for 2.0 hours. The reaction mixture was quenched with water. The aqueous layer was washed with dichloromethane (45 ml) at least ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com