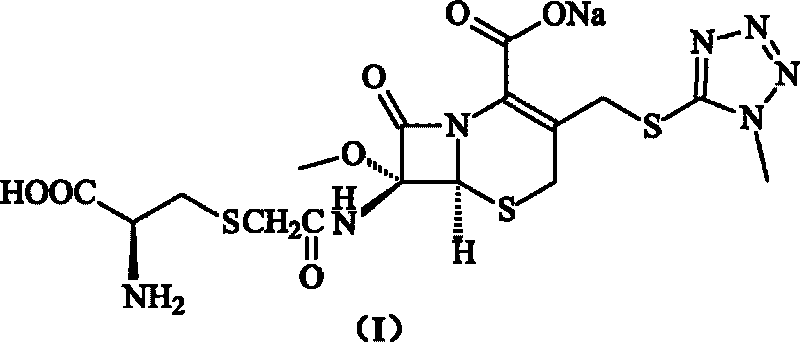
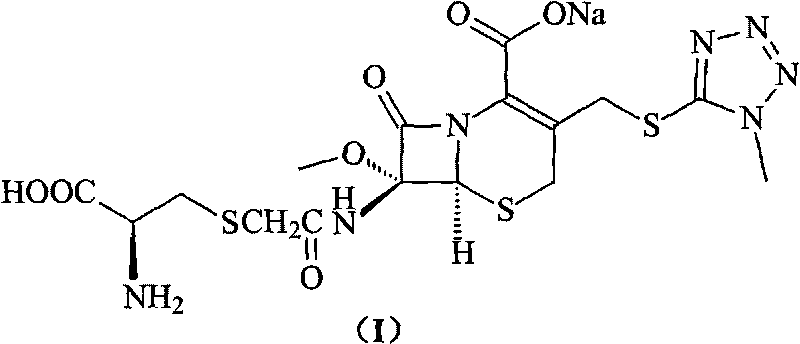
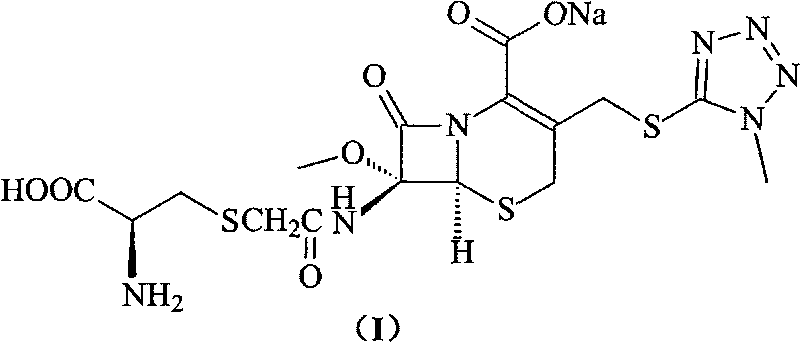
Cefminox sodium compound of new route
A technology of cefminox sodium and its compounds, which is applied in the field of drug synthesis, can solve the problems of large impurities, small toxic and side effects, and low purity, and achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 17
[0030] Example 17 Synthesis of β-bromoacetamido-7a-methoxyl group-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem-4-carboxylic acid
[0031]262 grams (0.5mol) of 7-MAC were dissolved in a solvent of 2 liters of acetone, and the reaction solution was cooled to 10°C, while 101 grams (0.5mol) of bromoacetyl bromide was dissolved in 800ml of acetone solution and 70ml of triethylamine. Add 800ml of acetone solution to the above reaction solution dropwise at the same time to keep the pH of the reaction system = 8. After the addition, keep the reaction at 10°C for 1 hour. This reaction is distilled off under reduced pressure at no more than 25°C. solvent, then the residue was added to 3 liters of ethyl acetate and stirred, then washed with 0.1mol / L hydrochloric acid, water, and 5% aqueous solution of sodium bicarbonate, dried with anhydrous sodium sulfate, filtered, and decompressed Concentrate to give 7β-bromoacetamido-7a-methoxyl group-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-c...
Embodiment 2
[0032] The synthesis of embodiment 2 cefminox sodium
[0033] 200 grams (0.42mol) of 7β-bromoacetamido-7a-methoxyl group-3-(1-methyl-1H-tetrazolium-5-thiomethyl)-3-cephem- 4-Carboxylic acid is suspended in water, adjust the pH=7.8 of reaction system with 10% sodium bicarbonate, add 5 grams of sodium iodide and 51 grams (0.42mol) of D-cysteine hydrochloride to this reaction solution , adjust the pH of the reaction system to 7.6 with 10% sodium bicarbonate, while heating, make the reaction react at 30°C for 1.5 hours, cool to room temperature, add 2L isopropanol, precipitate crystals, filter, and freeze-dry to obtain 193 grams of product , the yield was 85%; the purity detected by HPLC method was 99.8%.
[0034] Elemental Analysis C 16 h 20 N 7 NaO 7 S 3
[0035] Theoretical value C: 35.48%, H: 3.72%, N: 18.10%, O: 20.68%, S: 17.75%,
[0036] Experimental values C: 35.52%, H: 4.69%, N: 18.11%, O: 20.74%, S: 17.72%.
[0037] Through the chromatographic control test c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com