4'-demethylpodoph-yllotoxin derivative, its production and use
A technology for removing epipodophyllin and derivatives, which is applied in the field of synthesis of organic compounds, and can solve the problems of changing respiratory function, non-significant difference, damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
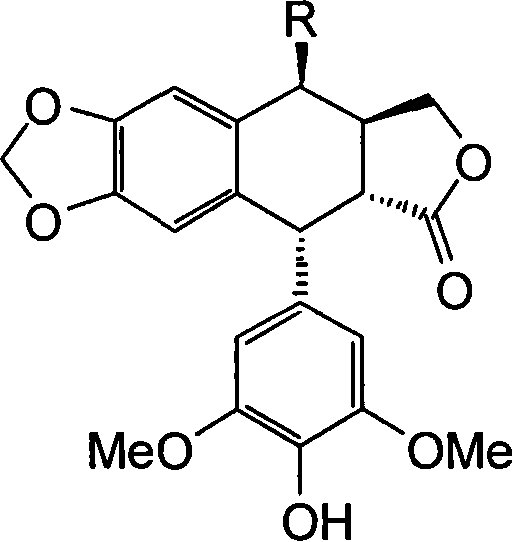
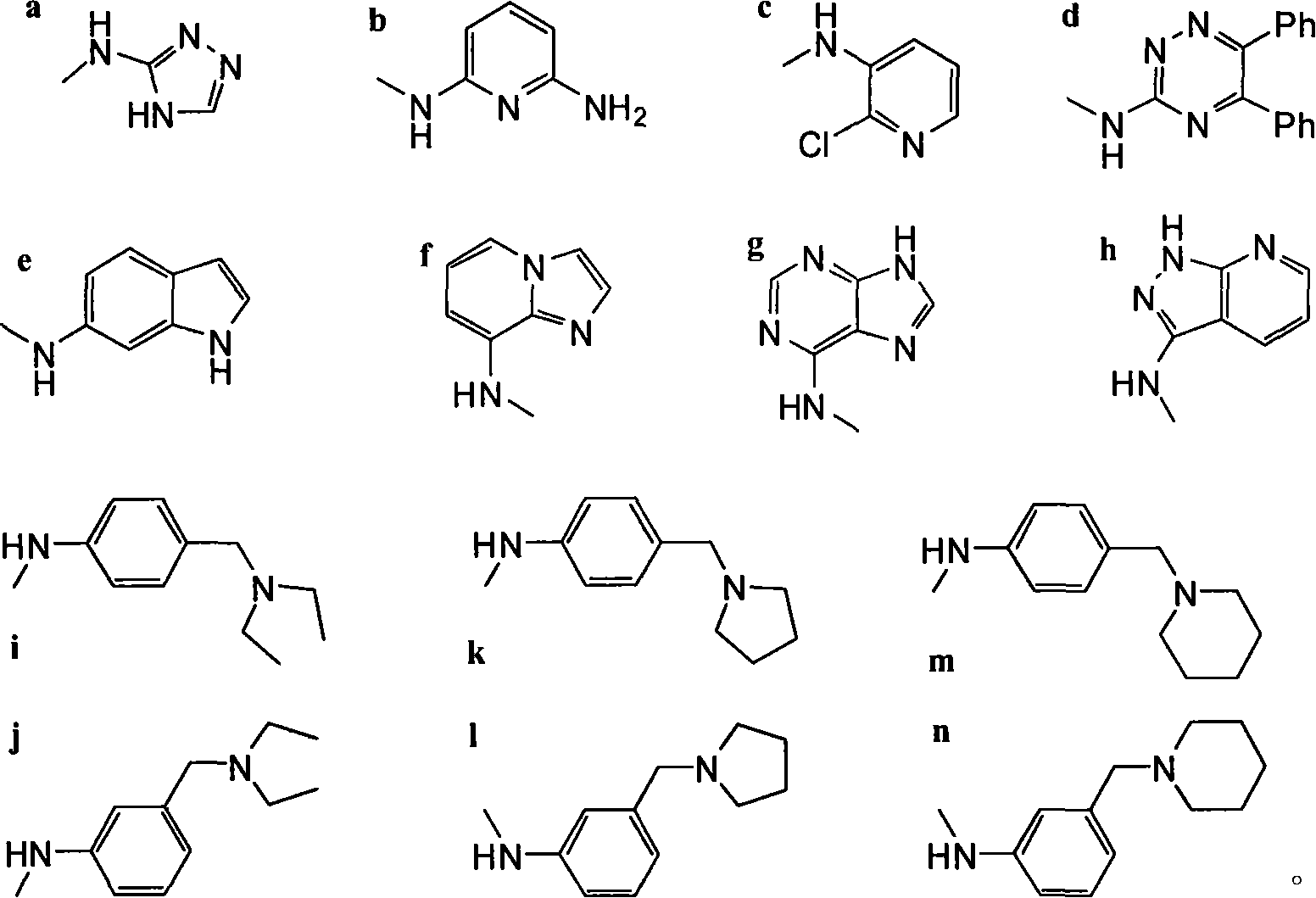
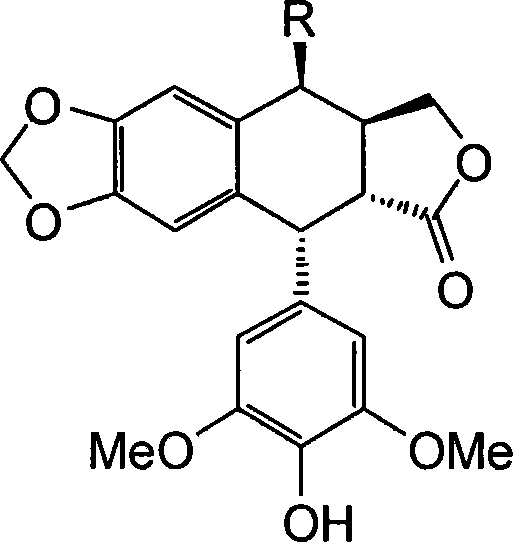
[0016] Example 1: Preparation of 4β-[N-(4H-1,2,4-triazol-4 base)]amino-4-deoxy-4'-norepipodophyllotoxin (compound IIIa)
[0017] Dissolve 88 mg (0.15 mmol) of etoposide and 90 mg (0.6 mmol) of sodium iodide in 1.5 mL of anhydrous acetonitrile, and stir at room temperature; in addition, dissolve 76 μL of dry trimethylchlorosilane in 1.5 mL of anhydrous acetonitrile, and This solution was slowly added dropwise to the reaction solution, stirred at room temperature for 1 hour to obtain the intermediate 4β-iodo-4-deoxy-4'-norepipodophyllotoxin (compound II), and 90 mg of anhydrous barium carbonate was added to the reaction solution (0.45mmol), stirred for 10 minutes, added a few drops of anhydrous triethylamine until the reaction solution was alkaline, added 3-amino-4H-1,2,4-triazole (0.18mmol), stirred at room temperature for 8-10 hours , suction filtration, the filtrate was concentrated under reduced pressure, the residue was dissolved in a small amount of dichloromethane, and pu...
Embodiment 2
[0019] Example 2: Preparation of 4β-[N-(6-aminopyridin-2 bases)]amino-4-deoxy-4'-norepipodophyllotoxin (compound IIIb)
[0020] The operation process is the same as in Example 1, except that 2,6-diaminopyridine is used instead of 3-amino-4H-1,2,4-triazole. 62 mg of yellow solid IIIb was obtained, yield: 84%. Melting point: 131-135°C.
[0021] 1 H NMR (400MHz, DMSO) δ8.27(brs, 1H, 4'-OH), 7.05(t, J=8.0Hz, 1H, pyridine-H), 6.83(s, 1H, H-5), 6.52( s, 1H, H-8), 6.45(d, J=8Hz, 1H, 4-NH), 6.26(s, 2H, H-2', H-6'), 5.97(d, J=11.6Hz, 2H, OCH 2 O), 5.69 (dd, J=17.6, 8.0Hz, 2H, pyridine-H), 5.45 (brs, 2H, NH 2 ), 5.37(m, 1H, H-4), 4.50(d, J=5.2Hz, 1H, H-1), 4.34(t, J=7.8Hz, 1H, H-11a), 3.74(m, 1H , H-11b), 3.64(s, 6H, 3', 5'-OCH 3 ), 3.26(dd, J=14.4, 5.2Hz, 1H, H-2), 2.91(m, 1H, H-3).
Embodiment 3
[0022] Example 3: Preparation of 4β-[N-(2-chloropyridin-3-yl)]amino-4-deoxy-4'-norepipodophyllotoxin (compound IIIc)
[0023] The operation process is the same as in Example 1, except that 3-amino-4H-1,2,4-triazole is replaced with 2-chloro-3-aminopyridine. 61 mg of yellow solid IIIc was obtained, yield: 80%. Melting point: 129-134°C.
[0024] 1H NMR (400MHz, DMSO) δ7.84 (brs, 1H, 4'-OH), 7.27 (s, 1H, pyridine-H), 7.17 (m, 1H, pyridine-H), 6.84 (m, 1H, pyridine -H), 6.74(s, 1H, H-5), 6.59(s, 1H, H-8), 6.34(s, 2H, H-2', H-6'), 5.99(m, 2H, OCH 2 O), 4.69(m, 2H, H-4, 4-NH), 4.57(m, 1H, H-1), 4.36(t, J=8.0Hz, 1H, H-11a), 3.87(m, 1H , H-11b), 3.80 (s, 6H, 3', 5'-OCH 3 ), 3.15(dd, J=14.4, 5.2Hz, 1H, H-2), 3.07(m, 1H, H-3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com