4'-demethylpodoph-yllotoxin derivative, its production and use
A technology for removing epipodophyllin and derivatives, which is applied in the field of synthesis of organic compounds, and can solve problems such as damage, hindering the efficacy of drugs, and inhibiting the dosage of drugs
Inactive Publication Date: 2010-11-24
ZHEJIANG UNIV
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Since TopoII is a cell cycle-dependent enzyme and a good substrate for P170 glycoprotein, tumor cells are more likely to develop drug resistance to TopoII than TopoI; in addition, cancer cells are transformed from normal cells, and the two types of cells The difference is not significant. While killing cancer cells, etoposide can damage normal cells in the body, especially bone marrow and intestinal mucosal cells that divide rapidly, resulting in myelosuppression, neutropenia, and nausea. , hyaline membrane disease and changes in normal respiratory function and other toxic and side effects, thereby inhibiting the dosage of the drug and hindering the efficacy of the drug
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
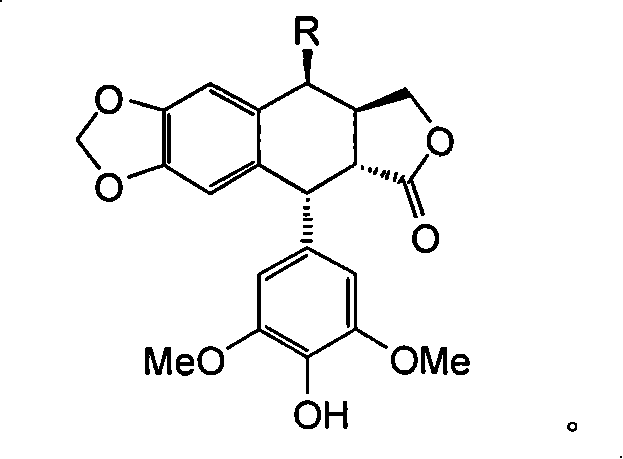
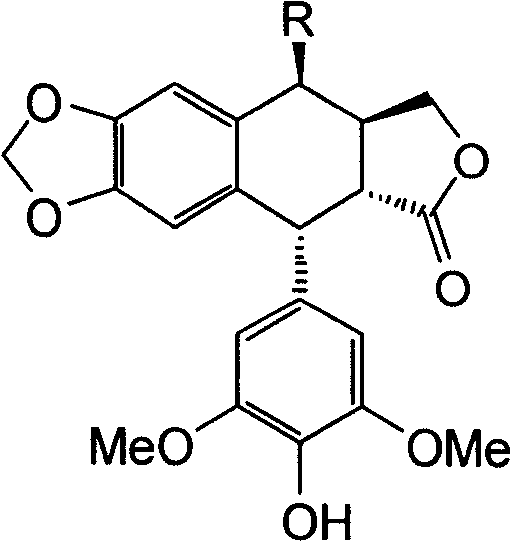
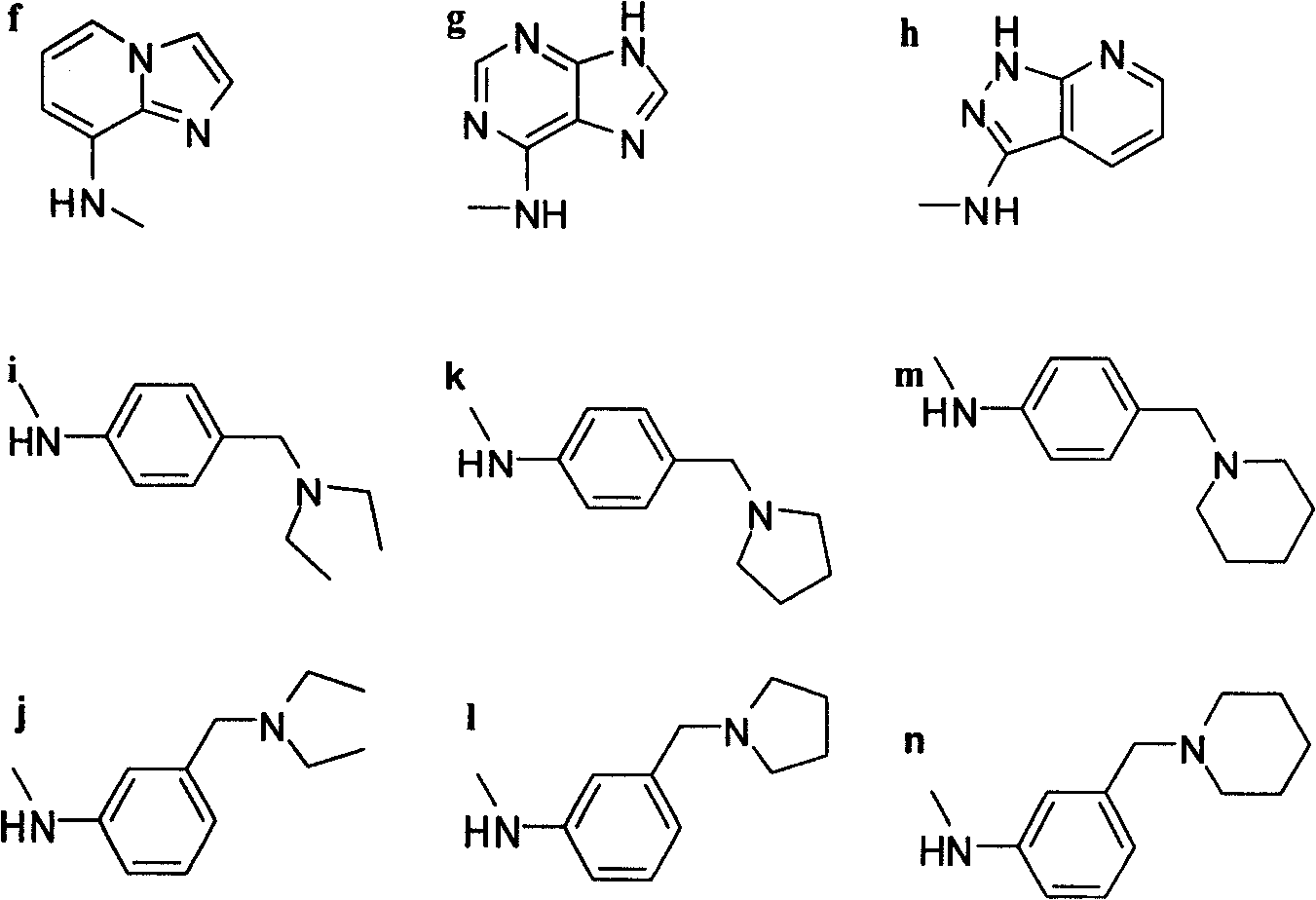
A 4'-demethylepipodophyllotoxin derivative IIIa-n is prepared by taking etoposide as raw material, reacting with sodium iodide to generate 4-bit iodine substitute and obtain intermediate II, agitating while adding into barium carbonate, regulating reactive system pH 7-8, adding into amino-compound, and reacting to room temperature for 8-10 hrs to obtain final product. It's simple and efficient, it can avoid 4-bit surface isomerization and 4-bit demethylation, it has strong inhibiting function of multiple tumor cell strain and non-toxic.
Description
4'-norepipodophyllotoxin derivative and its preparation method and use technical field The invention belongs to a synthesis method of organic compounds, and relates to a 4'-norepipodophyllotoxin derivative and a preparation method, mainly involving 4β-substituted amino-4-deoxy-4'-norepipodophyllotoxin derivatives and The preparation method, and the application in the preparation of antitumor drugs. Background technique Etoposide is a semi-synthetic derivative of the natural product podophyllotoxin. As a kind of glycoside compound with high anti-tumor activity, it is widely used in the clinical treatment of testicular cancer, lymphoma, leukemia, non-small cell lung cancer, acute myeloid leukemia and other cancers. treat. Etoposide acts as a TopoII inhibitor to exert antitumor effects. Since TopoII is a cell cycle-dependent enzyme and a good substrate for P170 glycoprotein, tumor cells are more likely to develop drug resistance to TopoII than TopoI; in addition, cancer ce...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D519/00A61K31/52A61K31/4196A61K31/443A61K31/4025C07D493/04A61P35/00A61K31/4525A61K31/437A61K31/404A61K31/53A61K31/357
Inventor 胡永洲杜文婷何俏军杨波杨晓春
Owner ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com