An improved synthesis of ceftiofur intermediate
A technology for cephem and aminocephalosporanic acid, which is applied in the directions of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., and can solve the problems of inability to obtain separation and poor stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment -I
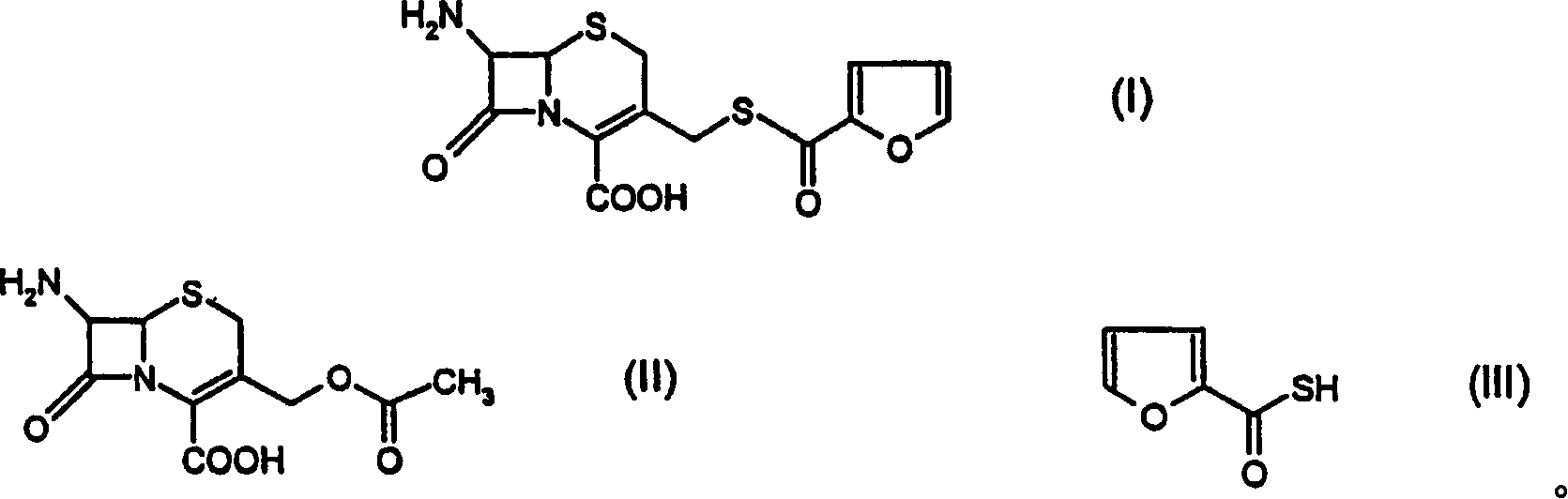
[0025] 7-Amino-3-(2-furylcarbonyl)thiomethyl]-3-cephem-4-carboxylic acid
[0026] Sodium sulfide (54.6 g) was added to water (600 ml), and furyl-2-carbonyl chloride (50.0 g) was added at a temperature of 20° C. within 1.0 hour. Ethyl acetate was added thereto, and the pH of the material was adjusted to 1.0 with hydrochloric acid. The organic layer was separated, dried over anhydrous sodium sulfate, and filtered to afford furyl-2-carbonylthiol in ethyl acetate.
[0027] Ethyl acetate (350 ml) was added to another flask, and boron trifluoride gas (124.0 g) was bubbled thereinto. 7-Amino-cephalosporanic acid (91.0 g) was added to the boron trifluoride solution at 10.0°C, followed by a solution of furyl-2-carbonylthiol in ethyl acetate (prepared above). After stirring at 30-40°C for 4-5 hours, the reaction was complete. After the reaction was complete, the mixture was poured into ice-cold water. Aqueous ammonia was added to adjust the pH of the solution to 3.45-3.55. The prec...
Embodiment -II
[0029] 7-Amino-3-(2-furylcarbonyl)thiomethyl]-3-cephem-4-carboxylic acid
[0030] Sodium sulfide (36.4 g) was added to water (400 ml), and furyl-2-carbonyl chloride (33.3.0 g) was added within 1.0 hour at a temperature of 20°C. Ethyl acetate was added thereto, and the pH of the material was adjusted to 1.0 with hydrochloric acid. The organic layer was separated, dried over anhydrous sodium sulfate, and filtered to afford furyl-2-carbonylthiol in ethyl acetate.
[0031] In another flask containing acetonitrile (350ml) was bubbled boron trifluoride gas (85.0g). 7-Amino-cephalosporanic acid (60.6 g) was added to the boron trifluoride solution at 10.0°C, followed by a solution of furyl-2-carbonylthiol in ethyl acetate (prepared above). After stirring at 30-40°C for 4-5 hours, the reaction was complete. After the reaction was complete, the material was poured into ice-cold water. Aqueous ammonia was added to adjust the pH of the solution to 3.45-3.55. The precipitated solid wa...
Embodiment -III
[0033] 7-Amino-3-(2-furylcarbonyl)thiomethyl]-3-cephem-4-carboxylic acid
[0034] Sodium sulfide (54.6 g) was added to water (600 ml), and furyl-2-carbonyl chloride (50.0 g) was added at a temperature of 20° C. within 1.0 hour. Ethyl acetate was added thereto, and the pH of the material was adjusted to 1.0 with hydrochloric acid. The organic layer was separated, dried over anhydrous sodium sulfate, and filtered to afford furyl-2-carbonylthiol in ethyl acetate.
[0035] To another flask containing acetonitrile (350ml) was added 7-amino-cephalosporanic acid (91.0g) at room temperature, followed by 45-48% boron trifluoride etherate (275.5ml) at 10.0°C. To this was added a solution of furyl-2-carbonylthiol in ethyl acetate (prepared above). After stirring at 40-50°C for 4-5 hours, the reaction was complete. After the reaction was completed, the reaction mixture was poured into ice-cold water. Sodium carbonate solution was added to adjust the pH of the solution to 3.45-3.55. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com