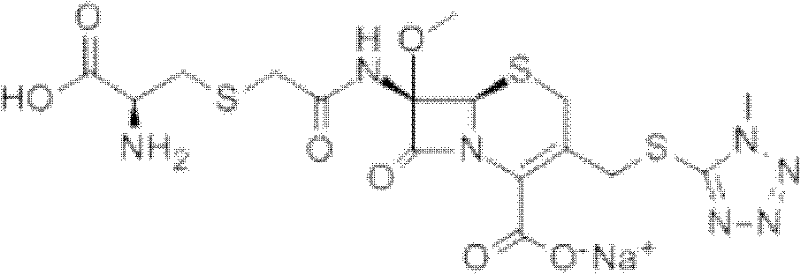
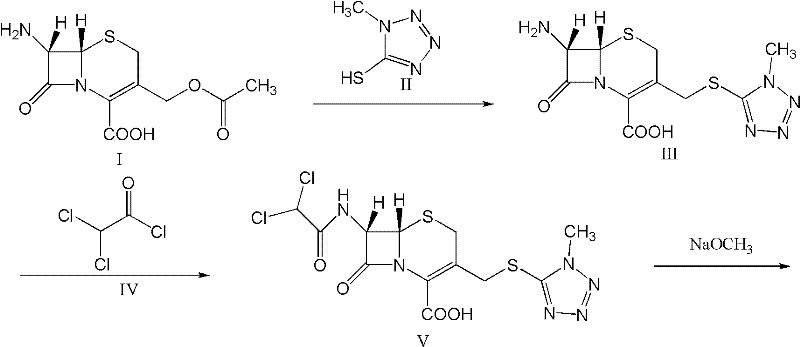
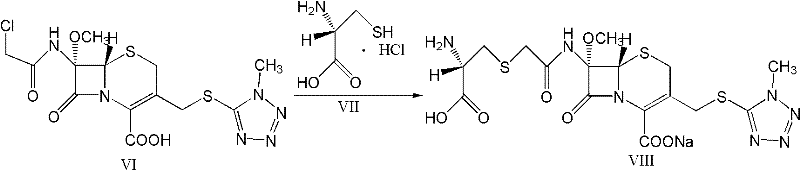
A kind of preparation method of cefminox sodium
A technology of cefminox sodium and cephem, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of expensive raw materials, low yield, and unsuitability for industrialized large-scale production, and achieves a small amount of impurity generation, a small amount of impurity generation, and finished products The effect of improving quality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Synthesis of 7β-amino-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid (3-TZ) at room temperature with 37.5g (0.138mol) 7-Aminocephalosporanic acid (7-ACA) and 16g (0.138mol) methylmercaptotetrazolium are mixed evenly with 200g acetonitrile as a solvent, (wet feeding is used to reduce the spread of 7-ACA dust and reduce the risk of 7-ACA allergy ). Add 100g of 20% boron trifluoride acetonitrile to the system and react at 35-40°C for 2 hours, add dropwise 20g of concentrated hydrochloric acid for crystallization, filter, wash with acetonitrile, and dry to obtain about 48.8g of intermediate 3-TZ with a yield of 130%.
[0056] 2. Synthesis of 7β-dichloroacetamido-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid (DCT)
[0057] Mix 33g (0.1mol) 3-TZ and 200g ethanol evenly at room temperature, add 17g dichloroacetyl chloride at 15-20°C, and react at the same temperature for 30 minutes. The liquid phase monitoring shows that the residual ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com