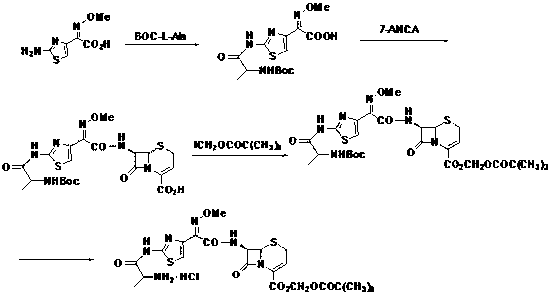
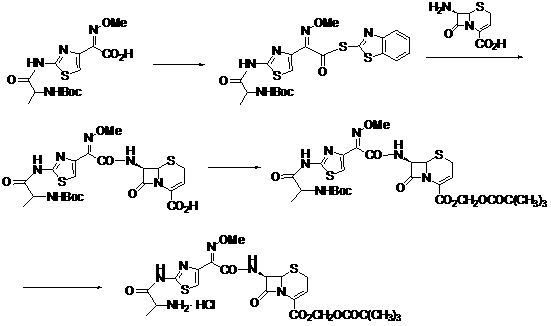
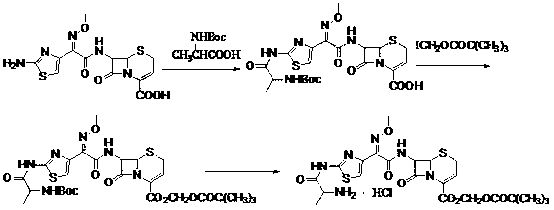
Method for preparing ceftizoxime alapivoxil hydrochloride
A technology of ceftizoxime propivoxil and hydrochloride, applied in the field of drug synthesis, can solve the problems of low product yield, unfavorable industrial production, and no literature reports yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (1) Preparation of formula III compound 7-N-tert-butoxycarbonyl-amino-3-cephem-4-carboxylic acid (7-Boc-ANCA)
[0072] 7-ANCA (4 g, 20 mmol) was dissolved in 50 ml of dioxane and water (1:1) mixed solvent, NaOH (1.2 g, 30 mmol) was added at 10 °C, and stirring was continued for 10 min. Join (Boc) 2 O (6.1 g, 28 mmol), reacted at 10 °C. TLC detection, after the reaction, add 50 ml of cold water to dilute, and use 4 mol L -1 After HCl was adjusted to pH = 2~3, it was extracted with cold ethyl acetate, then washed with 5% citric acid aqueous solution and saturated NaCl aqueous solution, and finally anhydrous NaCl was added. 2 SO 4 Let dry overnight. Concentrate under reduced pressure and recrystallize from dichloromethane and petroleum ether. 5.5 g of white crystals were obtained with a yield of 91.6%. Melting point 107~108℃; IR(KBr)ν(cm -1 ): 3315.97, 2979.81, 1790.33, 1750.12, 1638.93.
[0073] (2) Preparation of formula IV compound 7-N-tert-butoxycarbonylamin...
Embodiment 2
[0085] (1) Preparation of formula III compound 7-N-tert-butoxycarbonyl-amino-3-cephem-4-carboxylic acid (7-Boc-ANCA)
[0086] Dissolve 7-ANCA (4 g, 20 mmol) in 50 ml ethanol at about 0°C with stirring, add Na 2 CO 3 (2.6 g, 24 mmol), continue stirring for 10 min. Then join (Boc) 2 O (5.7 g, 26 mmol), reacted in an ice-water bath, and detected by TLC. After the reaction, concentrate under reduced pressure to recover the solvent, add 50 ml of cold water to dilute, and extract twice with cold dichloromethane; then adjust the pH of the aqueous phase to 2~3 in an ice-water bath, and then extract three times with cold dichloromethane; Combine the organic phases, wash with 5% citric acid aqueous solution, distilled water or saturated NaCl aqueous solution three times successively, add anhydrous NaCl 2 SO 4 Let dry overnight. Concentrate the solvent to crystallize, and then recrystallize with dichloromethane and petroleum ether. 5.73 g of white crystals were obtained with a y...
Embodiment 3
[0099] (1) Preparation of formula III compound 7-N-tert-butoxycarbonyl-amino-3-cephem-4-carboxylic acid (7-Boc-ANCA)
[0100] Add 7-ANCA (4 g, 20 mmol) into 50 ml of methanol, stir at about 5°C for 10 min, then drop triethylamine (4.2 ml, 30 mmol) and continue stirring for 10 min, add (Boc) 2 O (5.2 g, 24 mmol), the system was naturally warmed to room temperature and stirred for 12 h. The reaction solution was concentrated under reduced pressure to recover the solvent, diluted with 50 ml of cold distilled water, extracted twice with cold ethyl acetate, and then the water phase was adjusted to pH=2-3 in an ice bath, extracted three times with cold ethyl acetate, Combine the organic phases, wash with 5% citric acid aqueous solution, distilled water or saturated NaCl aqueous solution three times successively, add anhydrous NaCl 2 SO 4 Let dry overnight. The desiccant was filtered off, the solvent was evaporated under reduced pressure, and recrystallized directly with dichlor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com