Cefminox sodium raw material as well as preparation method and preparation thereof
A technology of cefminox sodium and cefminox sodium powder, which is applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem that cefminox sodium cannot be injected completely, Achieve the effects of small change in impurity content, simple preparation process, and small increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
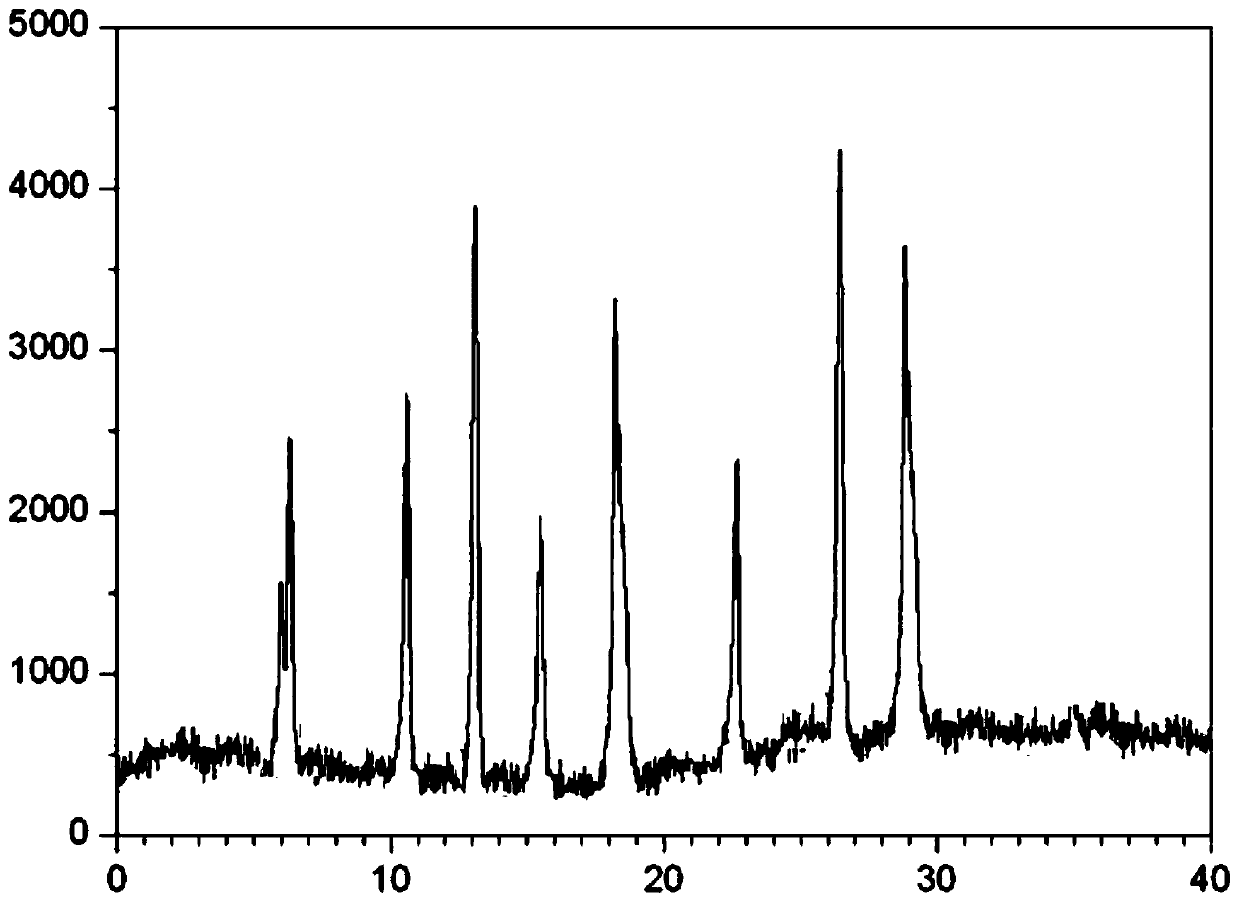
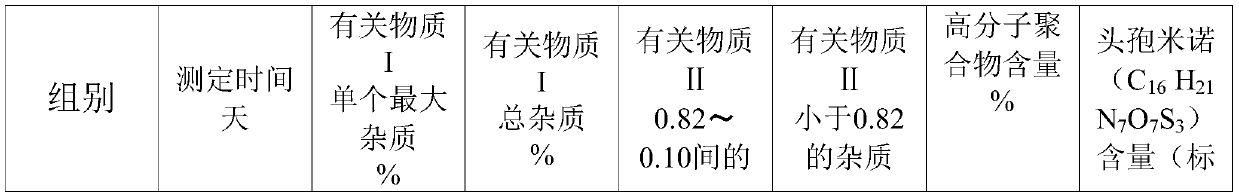
[0035] Take 100g of the crude product of cefminox sodium, add 350mL of tetrahydrofuran, heat up to 40°C, add 450mL of acetone, stir evenly, let it stand at 5°C-10°C, precipitate crystals, filter, wash the filter cake with acetone, and dry to obtain cefminox sodium The raw material is 78.2g; after testing, the residual solvent in the cefminox sodium raw material meets the Pharmacopoeia standard. After detection: (+)-(6R,7S)-7-[(S)-2-(2-amino-2-carboxyethylthio)acetamido]-7-methoxy-3-[[( l-Methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxy sodium heptahydrate. Calculated on the basis of anhydrous matter, containing cefminol (C 16 h 21 N 7 o 7 S 3 )99.7%. Related substance I: 0.014% for a single largest impurity, 0.078% for total impurities; Related substance II: 0.011% for impurities with a relative retention time between 0.82 and 0.10, and 0.011% for impurities with a relative retention time less than 0.82.
Embodiment 2
[0037] Take 100g of the crude product of cefminox sodium, add 500mL of tetrahydrofuran, heat up to 45°C, add 600mL of acetone, stir evenly, let it stand at 5°C-10°C, precipitate crystals, filter, wash the filter cake with acetone, and dry to obtain cefminox sodium Raw material 79.4g; After testing, the residual solvent in the cefminox sodium raw material complies with the Pharmacopoeia regulations. After detection: (+)-(6R,7S)-7-[(S)-2-(2-amino-2-carboxyethylthio)acetamido]-7-methoxy-3-[[( l-Methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxy sodium heptahydrate. Calculated on the basis of anhydrous matter, containing cefminol (C 16 h 21 N 7 o 7 S 3 )99.5%. Related substance I: the largest single impurity is 0.016%, and the total impurity is 0.124%; Related substance II: the impurity with a relative retention time between 0.82 and 0.10 is 0.014%, and the impurity with a relative retention time less than 0.82 is not detected.
Embodiment 3
[0039] Take 100g of the crude product of cefminox sodium, add 450mL of tetrahydrofuran, heat up to 50°C, add 550mL of acetone, stir evenly, let stand at 5°C-10°C, precipitate crystals, filter, wash the filter cake with acetone, and dry to obtain cefminox sodium Raw material 81.5g; After testing, the residual solvent in the cefminox sodium raw material complies with the Pharmacopoeia regulations. After detection: (+)-(6R,7S)-7-[(S)-2-(2-amino-2-carboxyethylthio)acetamido]-7-methoxy-3-[[( l-Methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxy sodium heptahydrate. Calculated on the basis of anhydrous matter, containing cefminol (C 16 h 21 N 7o 7 S 3 )99.8%. Related substance I: 0.010% for a single maximum impurity, 0.065% for total impurities; Related substance II: 0.011% for impurities with a relative retention time between 0.82 and 0.10, and 0.011% for impurities with a relative retention time less than 0.82.
[0040] 【Related Substan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com